Local Application of Isogenic Adipose-Derived Stem Cells Restores Bone Healing Capacity in a Type 2 Diabetes Model
- PMID: 27102648
- PMCID: PMC4878328
- DOI: 10.5966/sctm.2015-0158
Local Application of Isogenic Adipose-Derived Stem Cells Restores Bone Healing Capacity in a Type 2 Diabetes Model
Abstract
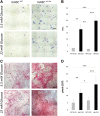
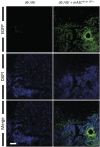
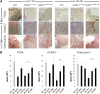
Bone regeneration is typically a reliable process without scar formation. The endocrine disease type 2 diabetes prolongs and impairs this healing process. In a previous work, we showed that angiogenesis and osteogenesis-essential steps of bone regeneration-are deteriorated, accompanied by reduced proliferation in type 2 diabetic bone regeneration. The aim of the study was to improve these mechanisms by local application of adipose-derived stem cells (ASCs) and facilitate bone regeneration in impaired diabetic bone regeneration. The availability of ASCs in great numbers and the relative ease of harvest offers unique advantages over other mesenchymal stem cell entities. A previously described unicortical tibial defect model was utilized in diabetic mice (Lepr(db-/-)). Isogenic mouse adipose-derived stem cells (mASCs)(db-/db-) were harvested, transfected with a green fluorescent protein vector, and isografted into tibial defects (150,000 living cells per defect). Alternatively, control groups were treated with Dulbecco's modified Eagle's medium or mASCs(WT). In addition, wild-type mice were identically treated. By means of immunohistochemistry, proteins specific for angiogenesis, cell proliferation, cell differentiation, and bone formation were analyzed at early (3 days) and late (7 days) stages of bone regeneration. Additionally, histomorphometry was performed to examine bone formation rate and remodeling. Histomorphometry revealed significantly increased bone formation in mASC(db-/db-)-treated diabetic mice as compared with the respective control groups. Furthermore, locally applied mASCs(db-/db-) significantly enhanced neovascularization and osteogenic differentiation. Moreover, bone remodeling was upregulated in stem cell treatment groups. Local application of mACSs can restore impaired diabetic bone regeneration and may represent a therapeutic option for the future.
Significance: This study showed that stem cells obtained from fat pads of type 2 diabetic mice are capable of reconstituting impaired bone regeneration in type 2 diabetes. These multipotent stem cells promote both angiogenesis and osteogenesis in type 2 diabetic bony defects. These data might prove to have great clinical implications for bony defects in the ever-increasing type 2 diabetic patient population.
Keywords: Bone; Diabetes mellitus; Fractures; Osteogenesis; Stem cells.
©AlphaMed Press.
Figures



References
-
- Gaston MS, Simpson AHRW. Inhibition of fracture healing. J Bone Joint Surg Br. 2007;89-B:1553–1560. - PubMed
-
- Retzepi M, Donos N. The effect of diabetes mellitus on osseous healing. Clin Oral Implants Res. 2010;21:673–681. - PubMed
-
- Daneman D. Type 1 diabetes. Lancet. 2006;367:847–858. - PubMed
-
- Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: eEstimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. - PubMed
-
- Isidro ML, Ruano B. Bone disease in diabetes. Curr Diabetes Rev. 2010;6:144–155. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

