Fas Regulates Macrophage Polarization and Fibrogenic Phenotype in a Model of Chronic Ethanol-Induced Hepatocellular Injury
- PMID: 27102767
- PMCID: PMC4901134
- DOI: 10.1016/j.ajpath.2016.02.006
Fas Regulates Macrophage Polarization and Fibrogenic Phenotype in a Model of Chronic Ethanol-Induced Hepatocellular Injury
Abstract
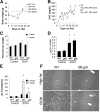
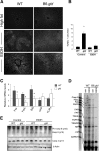
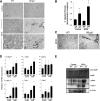
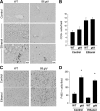
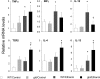
The role of Fas-mediated apoptosis and its effect on proinflammatory cytokine production in early alcoholic liver disease has not been addressed. Wild-type mice (C57Bl/6) or mice with a functional mutation in the Fas ligand (B6.gld) were given either high-fat control diet or ethanol diet by intragastric cannulation for 2 or 4 weeks. Liver injury, hepatic lipid accumulation, and proinflammatory cytokine production associated with chronic ethanol consumption were largely prevented in B6.gld mice compared with wild-type mice. Conversely, B6.gld mice given ethanol exhibited increases in collagen deposition, hepatic collagen gene expression, and profibrogenic cytokines (eg, transforming growth factor-β and IL-13) and alterations in matrix remodeling proteins (eg, matrix metalloproteinases and tissue inhibitor of metalloproteinases) compared with wild-type mice. Hepatic F4/80(+) macrophage populations were increased significantly in B6.gld mice compared with wild-type mice; hepatic CD3(+) cell populations were not significantly different. Importantly, a shift toward the expression of M2/Th2 cytokines (eg, IL-4 and IL-13) after ethanol exposure was observed in B6.gld mice compared with classical M1 cytokine expression in wild-type mice under similar conditions. In isolated macrophages, stimulation of Fas receptor minimally enhances lipopolysaccharide-induced M1 cytokine production and significantly limits M2 cytokine production. These data support the hypothesis that Fas-mediated signaling is important for an early ethanol-induced proinflammatory response but limits the profibrogenic response, regulating collagen production in response to chronic ethanol.
Copyright © 2016 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures

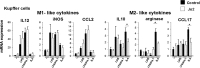
Similar articles
-
Death receptor response in rodent testis after mono-(2-ethylhexyl) phthalate exposure.Toxicol Appl Pharmacol. 2002 Dec 1;185(2):119-27. doi: 10.1006/taap.2002.9536. Toxicol Appl Pharmacol. 2002. PMID: 12490136
-
[Activation of Fas/FasL and its downstream signaling pathway promotes development of alcoholic steatohepatitis and liver fibrosis in mice].Zhonghua Gan Zang Bing Za Zhi. 2013 Feb;21(2):129-33. doi: 10.3760/cma.j.issn.1007-3418.2013.02.013. Zhonghua Gan Zang Bing Za Zhi. 2013. PMID: 23663886 Chinese.
-
Fas/Fas ligand-mediated apoptosis promotes hypersensitivity pneumonitis in mice by enhancing maturation of dendritic cells.Am J Respir Crit Care Med. 2010 Jun 1;181(11):1250-61. doi: 10.1164/rccm.200909-1337OC. Epub 2010 Mar 1. Am J Respir Crit Care Med. 2010. PMID: 20194815
-
Preimplantation maternal stress impairs embryo development by inducing oviductal apoptosis with activation of the Fas system.Mol Hum Reprod. 2016 Nov;22(11):778-790. doi: 10.1093/molehr/gaw052. Epub 2016 Jul 29. Mol Hum Reprod. 2016. PMID: 27475493
-
Role of ethanol in the regulation of hepatic stellate cell function.World J Gastroenterol. 2006 Nov 21;12(43):6926-32. doi: 10.3748/wjg.v12.i43.6926. World J Gastroenterol. 2006. PMID: 17109512 Free PMC article. Review.
Cited by
-
Somatic mutations in FAS pathway increase hemophagocytic lymphohistiocytosis risk in patients with T- and/or NK-cell lymphoma.Blood Adv. 2024 Jun 25;8(12):3064-3075. doi: 10.1182/bloodadvances.2023011733. Blood Adv. 2024. PMID: 38593227 Free PMC article.
-
Fas Ligand enhances vessel maturation and inhibits vascular leakage associated with age-related macular degeneration.Res Sq [Preprint]. 2024 May 8:rs.3.rs-4331250. doi: 10.21203/rs.3.rs-4331250/v1. Res Sq. 2024. PMID: 38766158 Free PMC article. Preprint.
-
Beneficial effects of an endogenous enrichment in n3-PUFAs on Wnt signaling are associated with attenuation of alcohol-mediated liver disease in mice.FASEB J. 2021 Feb;35(2):e21377. doi: 10.1096/fj.202001202R. FASEB J. 2021. PMID: 33481293 Free PMC article.
-
Macrophage Polarization and Its Role in Liver Disease.Front Immunol. 2021 Dec 14;12:803037. doi: 10.3389/fimmu.2021.803037. eCollection 2021. Front Immunol. 2021. PMID: 34970275 Free PMC article. Review.
-
The bidirectional immune crosstalk in metabolic dysfunction-associated steatotic liver disease.Cell Metab. 2023 Nov 7;35(11):1852-1871. doi: 10.1016/j.cmet.2023.10.009. Cell Metab. 2023. PMID: 37939656 Free PMC article. Review.
References
-
- Higuchi H., Gores G.J. Mechanisms of liver injury: an overview. Curr Mol Med. 2003;3:483–490. - PubMed
-
- Canbay A., Higuchi H., Bronk S.F., Taniai M., Sebo T.J., Gores G.J. Fas enhances fibrogenesis in the bile duct ligated mouse: a link between apoptosis and fibrosis. Gastroenterology. 2002;123:1323–1330. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

