Treatment with ActRIIB-mFc Produces Myofiber Growth and Improves Lifespan in the Acta1 H40Y Murine Model of Nemaline Myopathy
- PMID: 27102768
- PMCID: PMC4901141
- DOI: 10.1016/j.ajpath.2016.02.008
Treatment with ActRIIB-mFc Produces Myofiber Growth and Improves Lifespan in the Acta1 H40Y Murine Model of Nemaline Myopathy
Abstract
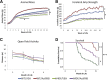
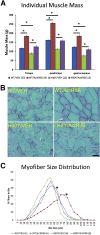
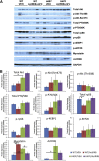
Nemaline myopathies (NMs) are a group of congenital muscle diseases caused by mutations in at least 10 genes and associated with a range of clinical symptoms. NM is defined on muscle biopsy by the presence of cytoplasmic rod-like structures (nemaline rods) composed of cytoskeletal material. Myofiber smallness is also found in many cases of NM and may represent a cause of weakness that can be counteracted by treatment. We have used i.p. injection of activin type IIB receptor (ActRIIB)-mFc (an inhibitor of myostatin signaling) to promote hypertrophy and increase strength in our prior murine work; we therefore tested whether ActRIIB-mFc could improve weakness in NM mice through myofiber hypertrophy. We report a study of ActRIIB-mFc treatment in the Acta1 H40Y mouse model of NM. Treatment of Acta1 H40Y mice produced significant increases in body mass, muscle mass, quadriceps myofiber size, and survival, but other measurements of strength (forelimb grip strength, ex vivo measurements of contractile function) did not improve. Our studies also identified that the complications of urethral obstruction are associated with mortality in male hemizygote Acta1 H40Y mice. The incidence of urethral obstruction and histologic evidence of chronic obstruction (inflammation) were significantly lower in Acta1 H40Y mice that had been treated with ActRIIB-mFc. ActRIIB-mFc treatment produces a mild benefit to the disease phenotype in Acta1 H40Y mice.
Copyright © 2016 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Myostatin Inhibition Using ActRIIB-mFc Does Not Produce Weight Gain or Strength in the Nebulin Conditional KO Mouse.J Neuropathol Exp Neurol. 2019 Feb 1;78(2):130-139. doi: 10.1093/jnen/nly120. J Neuropathol Exp Neurol. 2019. PMID: 30597051 Free PMC article.
-
Hypertrophy and dietary tyrosine ameliorate the phenotypes of a mouse model of severe nemaline myopathy.Brain. 2011 Dec;134(Pt 12):3516-29. doi: 10.1093/brain/awr274. Epub 2011 Nov 8. Brain. 2011. PMID: 22067542
-
Combined MRI and ³¹P-MRS investigations of the ACTA1(H40Y) mouse model of nemaline myopathy show impaired muscle function and altered energy metabolism.PLoS One. 2013 Apr 16;8(4):e61517. doi: 10.1371/journal.pone.0061517. Print 2013. PLoS One. 2013. PMID: 23613869 Free PMC article.
-
Clinical and Histologic Findings in ACTA1-Related Nemaline Myopathy: Case Series and Review of the Literature.Pediatr Neurol. 2017 Oct;75:11-16. doi: 10.1016/j.pediatrneurol.2017.04.002. Epub 2017 Apr 7. Pediatr Neurol. 2017. PMID: 28780987 Review.
-
Skeletal muscle α-actin diseases (actinopathies): pathology and mechanisms.Acta Neuropathol. 2013 Jan;125(1):19-32. doi: 10.1007/s00401-012-1019-z. Epub 2012 Jul 24. Acta Neuropathol. 2013. PMID: 22825594 Review.
Cited by
-
Spinal muscular atrophy-like phenotype in a mouse model of acid ceramidase deficiency.Commun Biol. 2023 May 25;6(1):560. doi: 10.1038/s42003-023-04932-w. Commun Biol. 2023. PMID: 37231125 Free PMC article.
-
Antimyostatin Treatment in Health and Disease: The Story of Great Expectations and Limited Success.Cells. 2021 Mar 3;10(3):533. doi: 10.3390/cells10030533. Cells. 2021. PMID: 33802348 Free PMC article. Review.
-
Pharmacological Inhibition of Myostatin in a Mouse Model of Typical Nemaline Myopathy Increases Muscle Size and Force.Int J Mol Sci. 2023 Oct 12;24(20):15124. doi: 10.3390/ijms242015124. Int J Mol Sci. 2023. PMID: 37894805 Free PMC article.
-
Sdha+/- Rats Display Minimal Muscle Pathology Without Significant Behavioral or Biochemical Abnormalities.J Neuropathol Exp Neurol. 2018 Aug 1;77(8):665-672. doi: 10.1093/jnen/nly042. J Neuropathol Exp Neurol. 2018. PMID: 29850869 Free PMC article.
-
Nebulin: big protein with big responsibilities.J Muscle Res Cell Motil. 2020 Mar;41(1):103-124. doi: 10.1007/s10974-019-09565-3. Epub 2020 Jan 25. J Muscle Res Cell Motil. 2020. PMID: 31982973 Free PMC article. Review.
References
-
- Wallgren-Pettersson C., Sewry C.A., Nowak K.J., Laing N.G. Nemaline myopathies. Semin Pediatr Neurol. 2011;18:230–238. - PubMed
-
- Gupta V.A., Ravenscroft G., Shaheen R., Todd E.J., Swanson L.C., Shiina M., Ogata K., Hsu C., Clarke N.F., Darras B.T., Farrar M.A., Hashem A., Manton N.D., Muntoni F., North K.N., Sandaradura S.A., Nishino I., Hayashi Y.K., Sewry C.A., Thompson E.M., Yau K.S., Brownstein C.A., Yu T.W., Allcock R.J., Davis M.R., Wallgren-Pettersson C., Matsumoto N., Alkuraya F.S., Laing N.G., Beggs A.H. Identification of KLHL41 mutations implicates BTB-Kelch-mediated ubiquitination as an alternate pathway to myofibrillar disruption in nemaline myopathy. Am J Hum Genet. 2013;93:1108–1117. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

