Loss of C9ORF72 impairs autophagy and synergizes with polyQ Ataxin-2 to induce motor neuron dysfunction and cell death
- PMID: 27103069
- PMCID: PMC4910533
- DOI: 10.15252/embj.201593350
Loss of C9ORF72 impairs autophagy and synergizes with polyQ Ataxin-2 to induce motor neuron dysfunction and cell death
Abstract
An intronic expansion of GGGGCC repeats within the C9ORF72 gene is the most common genetic cause of amyotrophic lateral sclerosis and frontotemporal dementia (ALS-FTD). Ataxin-2 with intermediate length of polyglutamine expansions (Ataxin-2 Q30x) is a genetic modifier of the disease. Here, we found that C9ORF72 forms a complex with the WDR41 and SMCR8 proteins to act as a GDP/GTP exchange factor for RAB8a and RAB39b and to thereby control autophagic flux. Depletion of C9orf72 in neurons partly impairs autophagy and leads to accumulation of aggregates of TDP-43 and P62 proteins, which are histopathological hallmarks of ALS-FTD SMCR8 is phosphorylated by TBK1 and depletion of TBK1 can be rescued by phosphomimetic mutants of SMCR8 or by constitutively active RAB39b, suggesting that TBK1, SMCR8, C9ORF72, and RAB39b belong to a common pathway regulating autophagy. While depletion of C9ORF72 only has a partial deleterious effect on neuron survival, it synergizes with Ataxin-2 Q30x toxicity to induce motor neuron dysfunction and neuronal cell death. These results indicate that partial loss of function of C9ORF72 is not deleterious by itself but synergizes with Ataxin-2 toxicity, suggesting a double-hit pathological mechanism in ALS-FTD.
Keywords: ALS‐FTD; C9ORF72; autophagy; neurodegeneration.
© 2016 The Authors.
Figures
Silver staining of proteins extracted from N2A mouse neuronal cells expressing Flag‐HA‐tagged C9ORF72 and captured through consecutive anti‐Flag and anti‐HA affinity purification steps.
Immunoblot analysis of HA‐immunoprecipitated proteins and lysate of HEK293 cells co‐expressing HA‐tagged C9ORF72 and/or Flag‐tagged SMCR8 and/or Flag‐tagged WDR41.
Coomassie blue staining of Nickel‐NTA affinity purification of HIS‐C9ORF72, SMCR8, and WDR41 co‐expressed in baculovirus‐infected insect cells.
Immunoblot analysis of HA‐immunoprecipitated proteins and lysate of HEK293 cells co‐expressing HA‐tagged C9ORF72 and HA‐tagged SMCR8 with various Flag‐tagged Rab GTPases.
Immunoblot against endogenous C9orf72, Wdr41, Rab8a, Rab39b, Rab5, and Rab7 of control (IgG alone) or endogenous Smcr8 immunoprecipitated from adult mouse brain.
α‐32P‐radiolabelled GDP release from GST‐tagged purified RAB8a as a function of increased concentration of either recombinant purified C9ORF72 alone or in complex with SMCR8 and WDR41.
Identical GDP release assay as in (F) but using recombinant purified GST‐tagged RAB39b instead of RAB8a.
Schematic representation of the C9ORF72 complex acting as a Rab‐guanine nucleotide exchange factor.

Immunoblot analysis of endogenous proteins found associated by proteomic analysis with control or Flag‐HA‐tagged C9ORF72 expressed in N2A cells.
Immunoblot analysis of HA‐immunoprecipitated proteins and lysate of HEK293 cells co‐expressing HA‐tagged long or short splicing variant of C9ORF72 with Flag‐tagged SMCR8 and Flag‐tagged WDR41.
Immunoblot analysis of HA‐immunoprecipitated proteins and lysate of HEK293 cells co‐expressing HA‐tagged C9ORF72 and/or HA‐tagged SMCR8 and/or HA‐tagged WDR41 with Flag‐tagged HSC70.
Left panel, immunoblot analysis of HA‐immunoprecipitated proteins and lysate of HEK293 cells co‐expressing either HA‐tagged C9ORF72, HA‐tagged SMCR8, or HA‐tagged WDR41 with Flag‐tagged RAB8A. Right panel, immunoblot analysis of HA‐immunoprecipitated proteins and lysate of HEK293 cells co‐expressing either HA‐tagged C9ORF72, HA‐tagged SMCR8, or HA‐tagged WDR41 with Flag‐tagged RAB39b.
Immunoblot analysis of HA‐immunoprecipitated proteins and lysate of HEK293 cells co‐expressing HA‐tagged SMCR8 with various Flag‐tagged Rab GTPases.
Immunoblot analysis of endogenous expression of Smcr8, Rab8a, Rab39b, and Gapdh in mouse adult tissues and primary culture of E18 cortical mouse neurons.
32P‐radiolabelled GDP release from GST‐tagged purified RAB8a, RAB39b, RAB29 (also known as RAB7L1), and RAB32 as a function of increased concentration of recombinant purified HIS‐C9ORF72:SMCR8:WDR41 complex purified from baculovirus‐infected insect cells. Error bars indicate SEM, N = 3.

Immunoblot analysis of HA‐immunoprecipitated proteins and lysate of HEK293 cells co‐expressing either HA‐tagged C9ORF72, HA‐tagged SMCR8, or HA‐tagged WDR41 with Flag‐tagged P62.
Immunoblot analysis of HA‐immunoprecipitated proteins and lysate of HEK293 cells co‐expressing either HA‐tagged C9ORF72, HA‐tagged SMCR8, or HA‐tagged WDR41 with Flag‐tagged OPTN.
Immunoblot analysis of HA‐immunoprecipitated proteins and lysate of HEK293 cells co‐expressing either HA‐tagged RAB8a or HA‐tagged RAB39b with Flag‐tagged P62.
Representative images of mouse GT1‐7 neuronal cells co‐transfected with GFP‐RFP‐LC3B and either control siRNA or siRNA targeting endogenous C9orf72 mRNA and treated or not with Torin.
Upper panel, immunoblot analysis of endogenous LC3B (Map1lc3b), C9orf72 and control Gapdh and actin of GT1‐7 neuronal cells transfected with either control siRNA or siRNA targeting C9orf72 mRNA and treated or not with Torin and/or bafilomycin A. Lower panel, real‐time RT–qPCR quantification of endogenous C9orf72 mRNA expression relative to Rplp0 mRNA.
Left panel, representative images of immunofluorescence labeling of endogenous P62 (Sqstm1) on GT1‐7 neuronal cells transfected with either control siRNA or siRNA targeting either C9orf72, Smcr8, or Wdr41 mRNA. Right panel, quantification of P62 aggregates.
Left panel, representative merged images of immunofluorescence labeling of transfected constructs (green) and endogenous P62 (Sqstm1, red) on GT1‐7 neuronal cells transfected with control siRNA or siRNA targeting C9orf72 and plasmids expressing control GFP or HA‐tagged long or short isoform of C9ORF72. Right panel, quantification of P62 aggregates.
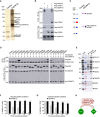
Left panel, representative images of organotypic cultures of E18 mouse cortical neurons transfected with GFP‐RFP‐LC3B and transduced with lentivirus expressing either control shRNA or shRNA targeting C9orf72 mRNA and treated or not with Torin. Right panel, quantification of LC3B puncta.
Upper panel, immunoblot analysis of endogenous LC3B (Map1lc3b), C9orf72, and control actin of E18 mouse cortical neurons transduced with lentivirus expressing either control shRNA or shRNA targeting C9orf72 mRNA and treated or not with Torin. Lower panel, real‐time RT–qPCR quantification of endogenous C9orf72 mRNA expression relative to Rplp0 mRNA.
Left panel, representative images of immunofluorescence labeling of endogenous P62 (Sqstm1) on organotypic cultures of E18 mouse cortical neurons transduced with lentiviral particles expressing either control shRNA or shRNA targeting C9orf72. Right panel, quantification of P62 aggregates.
Left panel, representative images of immunofluorescence labeling of transfected constructs (green) and endogenous P62 (Sqstm1, red) on GT1‐7 neuronal cells transfected with siRNA targeting C9orf72 and plasmids expressing control GFP, and HA‐tagged wild‐type or mutant RAB39b (CA, Q68L or CN, S22N), RAB8a, or RAB7. Right panel, quantification of P62 aggregates.

Immunoblot analysis of HA‐immunoprecipitated proteins and lysate of HEK293 cells co‐expressing HA‐tagged C9ORF72 and HA‐tagged SMCR8 with Flag‐tagged TBK1, NAP1, TANK, or SINTBAD.
Immunoprecipitated HA‐tagged C9ORF72, HA‐tagged SMCR8, and HA‐tagged WDR41 expressed in HEK293 were subjected to in vitro TBK1 kinase assay in the presence of γ‐32P‐radiolabelled ATP. Proteins were separated by SDS–PAGE migration and phosphorylation was detected by autoradiography (upper panel), while expression was detected by Western blotting (lower panel).
Mass spectrometry identification of SMCR8 phosphorylation sites by in vitro TBK1 kinase assay of HIS‐tagged C9ORF72:SMCR8:WDR41 complex purified from baculovirus‐infected insect cells.
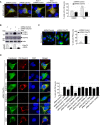
Upper panel, representative images of immunofluorescence labeling of transfected constructs (green) and endogenous P62 (Sqstm1, red) on GT1‐7 neuronal cells transfected with siRNA targeting the 3′UTR of Smcr8 mRNA and plasmids expressing control GFP and HA‐tagged wild‐type or mutant SMCR8 (TA, S402A and T796A; TD, S402D and T796D; UD, S400D, S492D, S562D, and T666D). Lower panel, quantification of P62 aggregates.
Upper panel, representative images of immunofluorescence labeling of transfected constructs (green) and endogenous P62 (Sqstm1, red) on GT1‐7 neuronal cells transfected with siRNA targeting Tbk1 and plasmids expressing control GFP and HA‐tagged wild‐type or mutant SMCR8 or RAB39b. Lower panel, quantification of P62 aggregates.
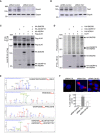
Immunoblot analysis of endogenous Smcr8 or Gapdh in GT1‐7 cells transfected with control siRNA or a siRNA targeting the 3′UTR of Smcr8.
Immunoblot analysis of endogenous Tbk1 or Gapdh in GT1‐7 cells transfected with control siRNA or a siRNA targeting Tbk1.
Immunoblot analysis of HA‐immunoprecipitated proteins and lysate of HEK293 cells co‐expressing HA‐tagged C9ORF72 and/or HA‐tagged SMCR8 and/or HA‐tagged WDR41 with Flag‐tagged ULK1.
HA‐tagged C9ORF72, HA‐tagged SMCR8, and HA‐tagged WDR41 were co‐expressed in HEK293, immunoprecipitated, and subjected to in vitro ULK1 kinase assay in the presence of γ‐32P‐radiolabelled ATP. Proteins were separated by migration on SDS–PAGE gel and phosphorylation was detected by autoradiography (upper panel), while expression was detected by Western blotting (lower panel).
Recombinant HIS‐tagged C9ORF72:SMCR8:WDR41 complex purified from baculovirus‐infected insect cells was phosphorylated in vitro by ULK1 and SMCR8 phosphorylation sites were identified by mass spectrometry.
Upper panel, representative images of immunofluorescence labeling of endogenous P62 (Sqstm1, red) on GT1‐7 neuronal cells transfected with control siRNA or siRNA targeting Tbk1 or Ulk1 and Ulk2. Lower panel, quantification of P62 aggregates.
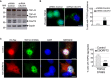
Immunoblot analysis of endogenous Tdp‐43, C9orf72, and control Gapdh of E18 mouse cortical neurons transduced with lentivirus expressing either control shRNA or shRNA targeting C9orf72 mRNA.
Left panel, representative images of immunofluorescence labeling of endogenous phosphorylated Ser409/410‐Tdp‐43 on primary cultures of E18 mouse cortical neurons transduced with lentiviral particles expressing either control shRNA or shRNA targeting C9orf72. Right panel, quantification of cytoplasmic Tdp‐43 aggregates.
Left panel, representative images of immunofluorescence labeling of D169G mutant GFP‐tagged TDP‐43 on primary cultures of E18 mouse cortical neurons transfected with either HA‐tagged control or HA‐C9ORF72 plasmid. Right panel, quantification of cells with cytoplasmic aggregates of TDP‐43.
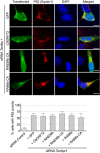
- A
Left panel, representative images of organotypic cultures of E18 mouse cortical neurons co‐transfected with HA‐tagged ATXN2 with control (Q22x) or intermediate (Q30x) polyQ size and transduced with lentivirus expressing either control shRNA or shRNA targeting C9orf72 mRNA. Right panel, quantification of Ataxin‐2 aggregates. Scale bars, 10 μm. Nuclei were counterstained with DAPI.
- B
Cell viability (tetrazolium assay) of GT1‐7 neuronal cells co‐transfected with HA‐tagged ATXN2 with control (Q22x) or intermediate (Q30x) polyQ size and control siRNA or siRNA targeting C9orf72 mRNA. Error bars indicate SEM, n = 3.
- C
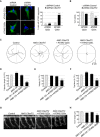
Tracing of the swimming trajectories of 48 h post‐fertilization zebrafish larvae following light touch.
- D–F
Quantification of the touch‐evoked swimming distance (D), average velocity (E), and maximum velocity attained (F) shows significant functional impairment of the zebrafish injected with HA‐tagged ATXN2 with intermediate length of polyQ (Q30x) and antisense morpholino oligonucleotides (AMOs) against C9orf72 compared to control HA‐tagged ATXN2 (Q22x) or to the sole injection of AMO against C9orf72.
- G
Representative images of motor neurons visualized with anti‐Sv2 immunohistochemistry show severe axonopathy in fish injected with both ATXN2 Q30x and AMO against C9orf72 compared to zebrafish injected with control ATXN2 Q22x or with AMO against C9orf72 alone.
- H
Quantification of the motor neuron axonal length demonstrates a significant decrease in axonal length in 48 h post‐fertilization zebrafish larvae injected with both ATXN2 Q30x and AMO against C9orf72 compared to controls.
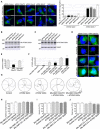
- A
Representative images of GT1‐7 neuronal cells co‐transfected with HA‐tagged ATXN2 with control (Q22x) or intermediate (Q30x) polyQ size and either control siRNA or siRNA targeting endogenous C9orf72 mRNA.
- B, C
Immunoblot analysis of endogenous Gapdh or transfected HA‐tagged Ataxin‐2 with (B) control (Q22x) length or (C) intermediate (Q30x) length of polyQ in GT1‐7 cells.
- D
Representative images of GT1‐7 neuronal cells co‐transfected with either GFP‐tagged mutant SOD1, FUS, HTT, or Ataxin‐3 and either control siRNA or siRNA targeting endogenous C9orf72 mRNA.
- E
RT–qPCR quantification of endogenous C9orf72 expression relative to Gapdh mRNA in mismatched or C9orf72 antisense morpholino oligonucleotide (AMO)‐injected zebrafish.
- F
RT–qPCR quantification of exogenous HA‐tagged ATXN2 with normal (Q22x) or intermediate (Q30x) length of polyQ relative to endogenous Gapdh mRNA in zebrafish injected with control or HA‐tagged ATXN2 constructs and with mismatched AMOs or AMOs against C9orf72.
- G
Tracing of the swimming trajectories of 48 h post‐fertilization zebrafish larvae following light touch.
- H–J
Quantification of the touch‐evoked swimming distance (H), average velocity (I), and maximum velocity attained (J) shows no impairment upon the sole injection of HA‐tagged ATXN2 with control (Q22x) or intermediate length of polyQ (Q30x). Similarly, injection of mismatched antisense morpholino oligonucleotides (AMOs) against C9orf72 alone or with HA‐tagged ATXN2 Q22x or Q30x shows no functional alterations.
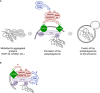
C9ORF72 in complex with SMCR8 and WDR41 acts as a GDP/GTP exchange factor for RAB39b GTPase, which interacts with the P62 autophagy receptor. Phosphorylation of SMCR8 by TBK1 potentially promotes C9ORF72 GEF activity and enhances autophagy turnover of proteins such as TDP‐43 or Ataxin‐2 with intermediate polyQ size. In the absence of C9ORF72, autophagy clearance of these proteins is reduced and TDP‐43, P62, or Ataxin‐2 with intermediate length of polyQ accumulates into cytoplasmic aggregates.
Autophagy receptors such as P62 or OPTN act as hubs to gather Rab GTPases with their GEF effectors and kinase regulators to initiate autophagy precisely at the site of damaged organelles, protein aggregates, or intracellular pathogens.
Comment in
-
Lost & found: C9ORF72 and the autophagy pathway in ALS/FTD.EMBO J. 2016 Jun 15;35(12):1251-3. doi: 10.15252/embj.201694578. Epub 2016 May 6. EMBO J. 2016. PMID: 27154207 Free PMC article.
References
-
- Almeida S, Gascon E, Tran H, Chou HJ, Gendron TF, Degroot S, Tapper AR, Sellier C, Charlet‐Berguerand N, Karydas A, Seeley WW, Boxer AL, Petrucelli L, Miller BL, Gao FB (2013) Modeling key pathological features of frontotemporal dementia with C9ORF72 repeat expansion in iPSC‐derived human neurons. Acta Neuropathol 126: 385–399 - PMC - PubMed
-
- Al‐Sarraj S, King A, Troakes C, Smith B, Maekawa S, Bodi I, Rogelj B, Al‐Chalabi A, Hortobágyi T, Shaw CE (2011) P62 positive, TDP‐43 negative, neuronal cytoplasmic and intranuclear inclusions in the cerebellum and hippocampus define the pathology of C9orf72‐linked FTLD and MND/ALS. Acta Neuropathol 122: 691–702 - PubMed
-
- Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, Oda T (2006) TDP‐43 is a component of ubiquitin‐positive tau‐negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun 351: 602–611 - PubMed
-
- Arndt V, Dick N, Tawo R, Dreiseidler M, Wenzel D, Hesse M, Fürst DO, Saftig P, Saint R, Fleischmann BK, Hoch M, Höhfeld J (2010) Chaperone‐assisted selective autophagy is essential for muscle maintenance. Curr Biol 20: 143–148 - PubMed
-
- Ash PE, Bieniek KF, Gendron TF, Caulfield T, Lin WL, van Blitterswijk MM, Jansen‐West K, Paul JW 3rd, Rademakers R, Boylan KB, Dickson DW, Petrucelli L (2013) Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron 77: 639–646 - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

