Aging is associated with dimerization and inactivation of the brain-enriched tyrosine phosphatase STEP
- PMID: 27103516
- PMCID: PMC4841919
- DOI: 10.1016/j.neurobiolaging.2016.02.004
Aging is associated with dimerization and inactivation of the brain-enriched tyrosine phosphatase STEP
Abstract
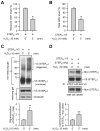
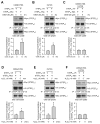
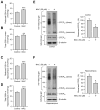
The STriatal-Enriched tyrosine Phosphatase (STEP) is involved in the etiology of several age-associated neurologic disorders linked to oxidative stress and is also known to play a role in neuroprotection by modulating glutamatergic transmission. However, the possible effect of aging on STEP level and activity in the brain is still unclear. In this study, using young (1 month), adult (4 months), and aged (18 months) rats, we show that aging is associated with increase in dimerization and loss of activity of STEP. Increased dimerization of STEP is primarily observed in the cortex and hippocampus and is associated with depletion of both reduced and total glutathione levels, suggesting an increase in oxidative stress. Consistent with this interpretation, studies in cell culture models of glutathione depletion and oxidative stress also demonstrate formation of dimers and higher order oligomers of STEP that involve intermolecular disulfide bond formation between multiple cysteine residues. Conversely, administration of N-acetyl cysteine, a major antioxidant that enhances glutathione biosynthesis, attenuates STEP dimerization both in the cortex and hippocampus. The findings indicate that loss of this intrinsic protective response pathway with age-dependent increase in oxidative stress may be a contributing factor for the susceptibility of the brain to age-associated neurologic disorders.
Keywords: Aging; Dimerization; Glutathione; N-acetyl cysteine; STEP; Tyrosine phosphatase.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


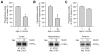


Similar articles
-
The Tyrosine Phosphatase STEP Is Involved in Age-Related Memory Decline.Curr Biol. 2018 Apr 2;28(7):1079-1089.e4. doi: 10.1016/j.cub.2018.02.047. Epub 2018 Mar 22. Curr Biol. 2018. PMID: 29576474 Free PMC article.
-
Oxidative stress-induced oligomerization inhibits the activity of the non-receptor tyrosine phosphatase STEP61.J Neurochem. 2011 Mar;116(6):1097-111. doi: 10.1111/j.1471-4159.2010.07165.x. Epub 2011 Jan 19. J Neurochem. 2011. PMID: 21198639 Free PMC article.
-
Down-regulation of gamma-glutamylcysteine synthetase regulatory subunit gene expression in rat brain tissue during aging.J Neurosci Res. 2002 May 1;68(3):344-51. doi: 10.1002/jnr.10217. J Neurosci Res. 2002. PMID: 12111865
-
Therapeutic implications for striatal-enriched protein tyrosine phosphatase (STEP) in neuropsychiatric disorders.Pharmacol Rev. 2012 Jan;64(1):65-87. doi: 10.1124/pr.110.003053. Epub 2011 Nov 16. Pharmacol Rev. 2012. PMID: 22090472 Free PMC article. Review.
-
[Indicators of oxidative stress in aging rat brain. The effect of nerve growth factor].Rev Neurol. 1998 Sep;27(157):494-500. Rev Neurol. 1998. PMID: 9774826 Review. Spanish.
Cited by
-
The Tyrosine Phosphatase STEP Is Involved in Age-Related Memory Decline.Curr Biol. 2018 Apr 2;28(7):1079-1089.e4. doi: 10.1016/j.cub.2018.02.047. Epub 2018 Mar 22. Curr Biol. 2018. PMID: 29576474 Free PMC article.
-
Amyloid β Modification: A Key to the Sporadic Alzheimer's Disease?Front Genet. 2017 May 15;8:58. doi: 10.3389/fgene.2017.00058. eCollection 2017. Front Genet. 2017. PMID: 28555154 Free PMC article. No abstract available.
-
Alterations of STEP46 and STEP61 Expression in the Rat Retina with Age and AMD-Like Retinopathy Development.Int J Mol Sci. 2020 Jul 22;21(15):5182. doi: 10.3390/ijms21155182. Int J Mol Sci. 2020. PMID: 32707818 Free PMC article.
-
Differential mRNA Expression Profiling Reveals the Role of MiR-375 in Inflammation of Bovine Mammary Epithelial Cells.Animals (Basel). 2022 Jun 1;12(11):1431. doi: 10.3390/ani12111431. Animals (Basel). 2022. PMID: 35681895 Free PMC article.
-
Emerging neuroprotective strategies for the treatment of ischemic stroke: An overview of clinical and preclinical studies.Exp Neurol. 2021 Jan;335:113518. doi: 10.1016/j.expneurol.2020.113518. Epub 2020 Nov 2. Exp Neurol. 2021. PMID: 33144066 Free PMC article. Review.
References
-
- Abe J, Okuda M, Huang Q, Yoshizumi M, Berk BC. Reactive oxygen species activate p90 ribosomal S6 kinase via Fyn and Ras. The Journal of biological chemistry. 2000;275:1739–1748. - PubMed
-
- Adam-Vizi V. Production of reactive oxygen species in brain mitochondria: contribution by electron transport chain and non-electron transport chain sources. Antioxidants & redox signaling. 2005;7:1140–1149. - PubMed
-
- Braak H, Braak E. Morphological criteria for the recognition of Alzheimer’s disease and the distribution pattern of cortical changes related to this disorder. Neurobiology of aging. 1994;15:355–356. discussion 379–380. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

