Reporting of harms outcomes: a comparison of journal publications with unpublished clinical study reports of orlistat trials
- PMID: 27103582
- PMCID: PMC4840982
- DOI: 10.1186/s13063-016-1327-z
Reporting of harms outcomes: a comparison of journal publications with unpublished clinical study reports of orlistat trials
Abstract
Background: The quality of harms reporting in journal publications is often poor, which can impede the risk-benefit interpretation of a clinical trial. Clinical study reports can provide more reliable, complete, and informative data on harms compared to the corresponding journal publication. This case study compares the quality and quantity of harms data reported in journal publications and clinical study reports of orlistat trials.
Methods: Publications related to clinical trials of orlistat were identified through comprehensive literature searches. A request was made to Roche (Genentech; South San Francisco, CA, USA) for clinical study reports related to the orlistat trials identified in our search. We compared adverse events, serious adverse events, and the reporting of 15 harms criteria in both document types and compared meta-analytic results using data from the clinical study reports against the journal publications.
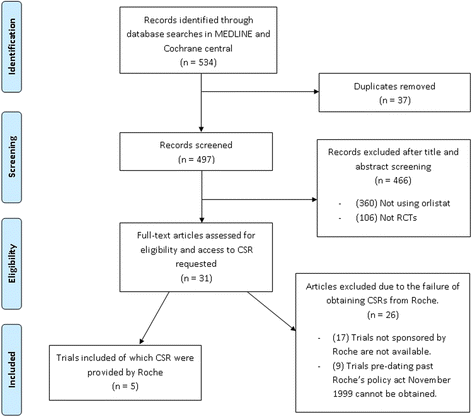
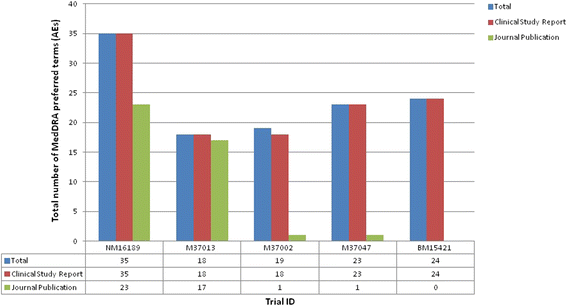
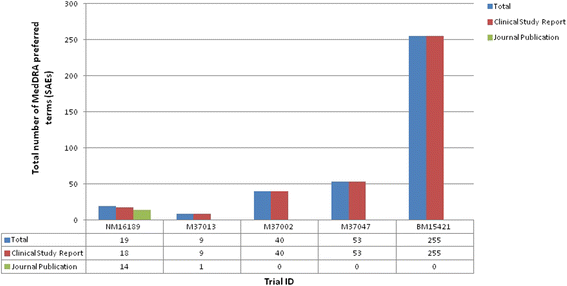
Results: Five journal publications with matching clinical study reports were available for five independent clinical trials. Journal publications did not always report the complete list of identified adverse events and serious adverse events. We found some differences in the magnitude of the pooled risk difference between both document types with a statistically significant risk difference for three adverse events and two serious adverse events using data reported in the clinical study reports; these events were of mild intensity and unrelated to the orlistat. The CONSORT harms reporting criteria were often satisfied in the methods section of the clinical study reports (70-90 % of the methods section criteria satisfied in the clinical study reports compared to 10-50 % in the journal publications), but both document types satisfied 80-100 % of the results section criteria, albeit with greater detail being provided in the clinical study reports.
Conclusions: In this case study, journal publications provided insufficient information on harms outcomes of clinical trials and did not specify that a subset of harms data were being presented. Clinical study reports often present data on harms, including serious adverse events, which are not reported or mentioned in the journal publications. Therefore, clinical study reports could support a more complete, accurate, and reliable investigation, and researchers undertaking evidence synthesis of harm outcomes should not rely only on incomplete published data that are presented in the journal publications.
Keywords: Adverse effect; Adverse event; Clinical study report; Evidence-based healthcare; Harms; Obesity; Orlistat; Randomised controlled trial; Systematic review.
Figures
References
-
- Wieseler B, Wolfram N, McGauran N, Kerekes MF, Vervölgyi V, Kohlepp P. Completeness of reporting of patient-relevant clinical trial outcomes: comparison of unpublished clinical study reports with publicly available data. PLoS Med. 2013;10(10):e1001526. doi: 10.1371/journal.pmed.1001526. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

