A randomized, blinded study to evaluate the efficacy and safety of umeclidinium 62.5 μg compared with tiotropium 18 μg in patients with COPD
- PMID: 27103795
- PMCID: PMC4827908
- DOI: 10.2147/COPD.S102494
A randomized, blinded study to evaluate the efficacy and safety of umeclidinium 62.5 μg compared with tiotropium 18 μg in patients with COPD
Abstract
Background: The long-acting muscarinic antagonists umeclidinium (UMEC) and tiotropium (TIO) are approved once-daily maintenance therapies for COPD. This study investigated the efficacy and safety of UMEC versus TIO in COPD.
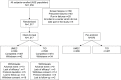
Methods: This was a 12-week, multicenter, randomized, blinded, double-dummy, parallel-group, non-inferiority study. Patients were randomized 1:1 to UMEC 62.5 μg plus placebo or TIO 18 μg plus placebo. The primary end point was trough forced expiratory volume in 1 second (FEV1) at day 85 (non-inferiority margin -50 mL; per-protocol [PP] population). Other end points included weighted mean FEV1 over 0-24 and 12-24 hours post-dose. Patient-reported outcomes comprised Transition Dyspnea Index score, St George's Respiratory Questionnaire total score, and COPD Assessment Test score. Adverse events were also assessed.
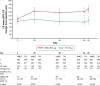
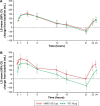
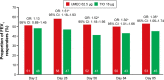
Results: In total, 1,017 patients were randomized to treatment. In the PP population, 489 and 487 patients received UMEC and TIO, respectively. In the PP population, change from baseline in trough FEV1 was greater with UMEC versus TIO at day 85, meeting non-inferiority and superiority margins (difference: 59 mL; 95% confidence interval [CI]: 29-88; P<0.001). Similar results were observed in the intent-to-treat analysis of trough FEV1 at day 85 (53 mL, 95% CI: 25-81; P<0.001). Improvements in weighted mean FEV1 over 0-24 hours post-dose at day 84 were similar with UMEC and TIO but significantly greater with UMEC versus TIO over 12-24 hours post-dose (70 mL; P=0.015). Clinically meaningful improvements in Transition Dyspnea Index and St George's Respiratory Questionnaire were observed with both treatments at all time points. No differences were observed between UMEC and TIO in patient-reported outcomes. Overall incidences of adverse events were similar for UMEC and TIO.
Conclusion: UMEC 62.5 μg demonstrated superior efficacy to TIO 18 μg on the primary end point of trough FEV1 at day 85. Safety profiles were similar for both treatments.
Trial registration: ClinicalTrials.gov NCT02207829.
Keywords: COPD; long-acting muscarinic antagonist; non-inferiority; tiotropium; umeclidinium.
Figures
References
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) [updated 2015] [Accessed August 12, 2015]. Available from: http://www.goldcopd.org/
-
- World Health Organization–burden of COPD. 2015. [Accessed August 19, 2015]. Available from: http://www.who.int/respiratory/copd/burden/en/
-
- Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543–1554. - PubMed
-
- O’Donnell DE, Fluge T, Gerken F, et al. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J. 2004;23(6):832–840. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

