Use of subcutaneous immunoglobulin in primary immune deficiencies
- PMID: 27103859
- PMCID: PMC4829171
- DOI: 10.5152/TurkPediatriArs.2016.3058
Use of subcutaneous immunoglobulin in primary immune deficiencies
Abstract
Aim: Immunoglobulin replacement therapy is required to reduce the frequency and severity of infections in patients with primary antibody deficiencies. Immunoglobulin G (IgG) can be administered intramuscularly, intravenously or subcutaneously. We aimed to evaluate the efficacy, dose adjustment and adverse events in subcutaneous immunoglobulin therapy by retrospectively presenting the records of 16 patients who received subcutaneous immunoglobulin therapy.
Material and methods: The demographic findings, clinical and laboratory findings, subcutaneous immunoglobulin dosage and dose frequency, infusion time, area and methods, adverse events and frequency of infections were obtained from patient files and recorded.
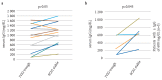
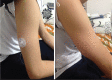
Results: Sixteen patients (seven female, nine male) aged between 0-33 years who were diagnosed with primary immune deficiency and treated with subcutaneous immunoglobulin were enrolled. All patients had been receiving intravenous imunoglobulin (5-10%) at a dose of 0.33-1.25 gr/kg/dose with two-four week intervals before subcutaneous immunoglobulin. Subcutaneous immunoglobulin (10%) was administered at a dose of 0.03-0.43 gr/kg/dose with one-two week intervals. No significant difference was found between serum through IgG levels before administration of intravenous imunoglobulin and steady state IgG levels during subcutaneous immunoglobulin therapy. When five patients whose serum through IgG levels were below 600 mg/dL were evaluated, however, a significant increase was found in steady state IgG levels with subcutaneous immunoglobulin therapy (p=0.043). In a ten-month follow-up period, seven infections were observed in four patients (three upper respiratory infectons, two lower respiratory tract infections and three acute gastroenteritis). No acute severe bacterial infection was observed. Local advers reaction was reported in only 10 of 180 infusions (6%). No serious adverse events were reported. All 16 patients were willing to continue IgG replacement therapy by subcutaneous administration.
Conclusions: Ig replacement therapy by subcutaneous route is an efficient, safe and easy option which is eligible for individual administration. Home therapy is feasible for patients with primary immune deficiency, if informed consent is obtained and sufficient education is ensured.
Keywords: Child; primary immune deficiency; subcutaneous immunoglobulin.
Figures
References
-
- Castigli E, Geha RS. Molecular basis of common variable immunodeficiency. J Allergy Clin Immunol. 2006;117:740–6. http://dx.doi.org/10.1016/j.jaci.2006.01.038. - DOI - PubMed
-
- Chapel HM. Consensus on diagnosis and management of primary antibody deficiencies. Consensus panel for the diagnosis and management of primary antibody deficiencies. BMJ. 1994;308:581–5. http://dx.doi.org/10.1136/bmj.308.6928.581. - DOI - PMC - PubMed
-
- Notarangelo L, Casanova JL, Fischer A, et al. International union of immunological societies primary immunodeficiency diseases classification committee. Primary immunodeficiency diseases: an update. J Allergy Clin Immunol. 2004;114:677–87. http://dx.doi.org/10.1016/j.jaci.2004.06.044. - DOI - PMC - PubMed
-
- Moore ML, Quinn JM. Subcutaneous immunoglobulin replacement therapy for primary antibody deficiency: advancements into the 21st century. Ann Allergy Asthma Immunol. 2008;101:114–21. http://dx.doi.org/10.1016/S1081-1206(10)60197-4. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources