Rearrangements of Cycloalkenyl Aryl Ethers
- PMID: 27104504
- PMCID: PMC6273085
- DOI: 10.3390/molecules21040503
Rearrangements of Cycloalkenyl Aryl Ethers
Abstract
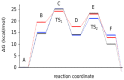
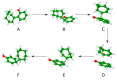
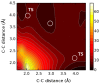
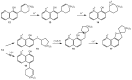
Rearrangement reactions of cycloalkenyl phenol and naphthyl ethers and the acid-catalyzed cyclization of the resulting product were investigated. Claisen rearrangement afforded 2-substituted phenol and naphthol derivatives. Combined Claisen and Cope rearrangement resulted in the formation of 4-substituted phenol and naphthol derivatives. In the case of cycloocthylphenyl ether the consecutive Claisen and Cope rearrangements were followed by an alkyl migration. The mechanism of this novel rearrangement reaction is also discussed.
Keywords: Claisen rearrangement; Cope rearrangement; naphthyl ethers; phenol ethers; reaction mechanism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Stereocontrolled synthesis of contiguous C(sp3)-C(aryl) bonds by lanthanide(III)-catalyzed domino aryl-Claisen [3,3]-sigmatropic rearrangements.Org Lett. 2010 Oct 15;12(20):4446-9. doi: 10.1021/ol1018147. Org Lett. 2010. PMID: 20857953
-
Synthesis of functionalized furans via gold(I)-catalyzed Claisen-type rearrangement.J Org Chem. 2008 Jan 18;73(2):730-3. doi: 10.1021/jo7022685. Epub 2007 Dec 13. J Org Chem. 2008. Retraction in: J Org Chem. 2008 Mar 7;73(5):2032. doi: 10.1021/jo800265n. PMID: 18076190 Retracted.
-
Chiral cobalt(ii) complex-promoted asymmetric para-Claisen rearrangement of allyl α-naphthol ethers.Chem Sci. 2023 Nov 27;14(47):13979-13985. doi: 10.1039/d3sc05677e. eCollection 2023 Dec 6. Chem Sci. 2023. PMID: 38075639 Free PMC article.
-
Radical addition-initiated domino reactions of conjugated oxime ethers.Chem Pharm Bull (Tokyo). 2014;62(9):845-55. doi: 10.1248/cpb.c14-00388. Chem Pharm Bull (Tokyo). 2014. PMID: 25177013 Review.
-
Phenols in Pharmaceuticals: Analysis of a Recurring Motif.J Med Chem. 2022 May 26;65(10):7044-7072. doi: 10.1021/acs.jmedchem.2c00223. Epub 2022 May 9. J Med Chem. 2022. PMID: 35533692 Review.
References
-
- Pereira D.M., Valentao P., Pereira J.A., Andrade P.B. Fenolics: From chemistry to biology. Molecules. 2009;14:2202–2211. doi: 10.3390/molecules14062202. - DOI
-
- Harbor J.B. Biochemistry of Phenolic Compounds. Academic Press; London, UK: 1964.
-
- Vermeries W., Nicolson R. Biochemistry of Phenolic Compounds. Springer; Heidelberg, Germany: 2006.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

