Deregulation of Rho GTPases in cancer
- PMID: 27104658
- PMCID: PMC5003542
- DOI: 10.1080/21541248.2016.1173767
Deregulation of Rho GTPases in cancer
Abstract
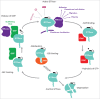
In vitro and in vivo studies and evidence from human tumors have long implicated Rho GTPase signaling in the formation and dissemination of a range of cancers. Recently next generation sequencing has identified direct mutations of Rho GTPases in human cancers. Moreover, the effects of ablating genes encoding Rho GTPases and their regulators in mouse models, or through pharmacological inhibition, strongly suggests that targeting Rho GTPase signaling could constitute an effective treatment. In this review we will explore the various ways in which Rho signaling can be deregulated in human cancers.
Keywords: GAPs; GDI; GEFs; Rho GTPases mutations; cancer; tumorigenesis.
Figures

References
-
- Hall A. Rho family GTPases. Biochem Soc Trans 2012; 40:1378-82; PMID:23176484; http://dx.doi.org/10.1042/BST20120103 - DOI - PubMed
-
- Orgaz JL, Herraiz C, Sanz-Moreno V. Rho GTPases modulate malignant transformation of tumor cells. Small GTPases 2014; 5:e29019; PMID:25036871; http://dx.doi.org/10.4161/sgtp.29019 - DOI - PMC - PubMed
-
- Sadok A, Marshall CJ. Rho GTPases: masters of cell migration. Small GTPases 2014; 5:e29710; PMID:24978113; http://dx.doi.org/10.4161/sgtp.29710 - DOI - PMC - PubMed
-
- Knaus UG, Heyworth PG, Evans T, Curnutte JT, Bokoch GM. Regulation of phagocyte oxygen radical production by the GTP-binding protein Rac 2. Science 1991; 254:1512-5; PMID:1660188; http://dx.doi.org/10.1126/science.1660188 - DOI - PubMed
-
- Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol 2005; 21:247-69; PMID:16212495; http://dx.doi.org/10.1146/annurev.cellbio.21.020604.150721 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
