Alterations of Dermal Connective Tissue Collagen in Diabetes: Molecular Basis of Aged-Appearing Skin
- PMID: 27104752
- PMCID: PMC4841569
- DOI: 10.1371/journal.pone.0153806
Alterations of Dermal Connective Tissue Collagen in Diabetes: Molecular Basis of Aged-Appearing Skin
Abstract
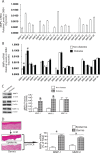
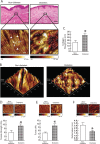
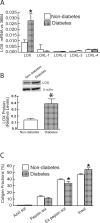
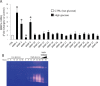
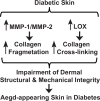
Alterations of the collagen, the major structural protein in skin, contribute significantly to human skin connective tissue aging. As aged-appearing skin is more common in diabetes, here we investigated the molecular basis of aged-appearing skin in diabetes. Among all known human matrix metalloproteinases (MMPs), diabetic skin shows elevated levels of MMP-1 and MMP-2. Laser capture microdissection (LCM) coupled real-time PCR indicated that elevated MMPs in diabetic skin were primarily expressed in the dermis. Furthermore, diabetic skin shows increased lysyl oxidase (LOX) expression and higher cross-linked collagens. Atomic force microscopy (AFM) further indicated that collagen fibrils were fragmented/disorganized, and key mechanical properties of traction force and tensile strength were increased in diabetic skin, compared to intact/well-organized collagen fibrils in non-diabetic skin. In in vitro tissue culture system, multiple MMPs including MMP-1 and MM-2 were induced by high glucose (25 mM) exposure to isolated primary human skin dermal fibroblasts, the major cells responsible for collagen homeostasis in skin. The elevation of MMPs and LOX over the years is thought to result in the accumulation of fragmented and cross-linked collagen, and thus impairs dermal collagen structural integrity and mechanical properties in diabetes. Our data partially explain why old-looking skin is more common in diabetic patients.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

