Characterization and Clinical Impact of Bloodstream Infection Caused by Carbapenemase-Producing Enterobacteriaceae in Seven Latin American Countries
- PMID: 27104910
- PMCID: PMC4841576
- DOI: 10.1371/journal.pone.0154092
Characterization and Clinical Impact of Bloodstream Infection Caused by Carbapenemase-Producing Enterobacteriaceae in Seven Latin American Countries
Abstract
Introduction: Infections caused by carbapenem-resistant Enterobacteriaceae are a public health problem associated with higher mortality rates, longer hospitalization and increased healthcare costs. We carried out a study to describe the characteristics of patients with carbapenemase-producing Enterobacteriaceae (CPE) and non-CPE bloodstream infection (BSI) from Latin American hospitals and to determine the clinical impact in terms of mortality and antibiotic therapy.
Methods: Between July 2013 and November 2014, we conducted a multicenter observational study in 11 hospitals from 7 Latin American countries (Argentina, Colombia, Ecuador, Guatemala, Mexico, Peru, Venezuela). Patients with BSI caused by Enterobacteriaceae were included and classified either as CPE or non-CPE based on detection of blaKPC, blaVIM, blaIMP, blaNDM and blaOXA-48 by polymerase chain reaction. Enrolled subjects were followed until discharge or death. Demographic, microbiological and clinical characteristics were collected from medical records. Both descriptive and inferential statistics were used to analyze the information.
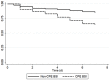
Results: A total of 255 patients with Enterobacteriaceae BSI were included; CPE were identified in 53 of them. In vitro non-susceptibility to all screened antibiotics was higher in the patients with CPE BSI, remaining colistin, tigecycline and amikacin as the most active drugs. Combination therapy was significantly more frequent in the CPE BSI group (p < 0.001). The most common regimen was carbapenem + colistin or polymyxin B. The overall mortality was 37% (94/255). Overall and attributable mortality were significantly higher in patients with CPE BSI (p < 0.001); however, we found that patients with CPE BSI who received combination therapy and those who received monotherapy had similar mortality. After multivariate adjustment, CPE BSI (adjusted odds ratio [aOR] 4; 95% confidence interval [CI] 1.7-9.5; p = 0.002) and critical illness (aOR 6.5; 95% CI 3.1-13.7; p < 0.001) were independently associated with in-hospital mortality.
Conclusions: This study provides valuable data on the clinical characteristics and mortality risk factors in patients with CPE BSI. We determined that CPE infection is an independent mortality predictor and thus Latin American hospitals should perform campaigns on prevention and control of CPE BSI.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous