The initiator caspase Dronc is subject of enhanced autophagy upon proteasome impairment in Drosophila
- PMID: 27104928
- PMCID: PMC5072431
- DOI: 10.1038/cdd.2016.40
The initiator caspase Dronc is subject of enhanced autophagy upon proteasome impairment in Drosophila
Abstract
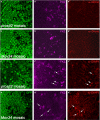
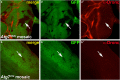
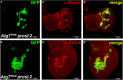
A major function of ubiquitylation is to deliver target proteins to the proteasome for degradation. In the apoptotic pathway in Drosophila, the inhibitor of apoptosis protein 1 (Diap1) regulates the activity of the initiator caspase Dronc (death regulator Nedd2-like caspase; caspase-9 ortholog) by ubiquitylation, supposedly targeting Dronc for degradation by the proteasome. Using a genetic approach, we show that Dronc protein fails to accumulate in epithelial cells with impaired proteasome function suggesting that it is not degraded by the proteasome, contrary to the expectation. Similarly, decreased autophagy, an alternative catabolic pathway, does not result in increased Dronc protein levels. However, combined impairment of the proteasome and autophagy triggers accumulation of Dronc protein levels suggesting that autophagy compensates for the loss of the proteasome with respect to Dronc turnover. Consistently, we show that loss of the proteasome enhances endogenous autophagy in epithelial cells. We propose that enhanced autophagy degrades Dronc if proteasome function is impaired.
Figures



References
-
- Pickart CM, Cohen RE. Proteasomes and their kin: proteases in the machine age. Nat Rev Mol Cell Biol 2004; 5: 177–187. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

