Fasciola hepatica Surface Coat Glycoproteins Contain Mannosylated and Phosphorylated N-glycans and Exhibit Immune Modulatory Properties Independent of the Mannose Receptor
- PMID: 27104959
- PMCID: PMC4841591
- DOI: 10.1371/journal.pntd.0004601
Fasciola hepatica Surface Coat Glycoproteins Contain Mannosylated and Phosphorylated N-glycans and Exhibit Immune Modulatory Properties Independent of the Mannose Receptor
Abstract
Fascioliasis, caused by the liver fluke Fasciola hepatica, is a neglected tropical disease infecting over 1 million individuals annually with 17 million people at risk of infection. Like other helminths, F. hepatica employs mechanisms of immune suppression in order to evade its host immune system. In this study the N-glycosylation of F. hepatica's tegumental coat (FhTeg) and its carbohydrate-dependent interactions with bone marrow derived dendritic cells (BMDCs) were investigated. Mass spectrometric analysis demonstrated that FhTeg N-glycans comprised mainly of oligomannose and to a lesser extent truncated and complex type glycans, including a phosphorylated subset. The interaction of FhTeg with the mannose receptor (MR) was investigated. Binding of FhTeg to MR-transfected CHO cells and BMDCs was blocked when pre-incubated with mannan. We further elucidated the role played by MR in the immunomodulatory mechanism of FhTeg and demonstrated that while FhTeg's binding was significantly reduced in BMDCs generated from MR knockout mice, the absence of MR did not alter FhTeg's ability to induce SOCS3 or suppress cytokine secretion from LPS activated BMDCs. A panel of negatively charged monosaccharides (i.e. GlcNAc-4P, Man-6P and GalNAc-4S) were used in an attempt to inhibit the immunoregulatory properties of phosphorylated oligosaccharides. Notably, GalNAc-4S, a known inhibitor of the Cys-domain of MR, efficiently suppressed FhTeg binding to BMDCs and inhibited the expression of suppressor of cytokine signalling (SOCS) 3, a negative regulator the TLR and STAT3 pathway. We conclude that F. hepatica contains high levels of mannose residues and phosphorylated glycoproteins that are crucial in modulating its host's immune system, however the role played by MR appears to be limited to the initial binding event suggesting that other C-type lectin receptors are involved in the immunomodulatory mechanism of FhTeg.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



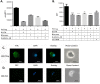
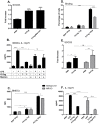
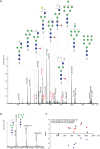
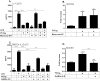
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

