Legionella pneumophila-Derived Outer Membrane Vesicles Promote Bacterial Replication in Macrophages
- PMID: 27105429
- PMCID: PMC4841580
- DOI: 10.1371/journal.ppat.1005592
Legionella pneumophila-Derived Outer Membrane Vesicles Promote Bacterial Replication in Macrophages
Abstract
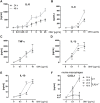
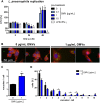
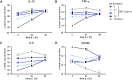
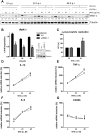
The formation and release of outer membrane vesicles (OMVs) is a phenomenon of Gram-negative bacteria. This includes Legionella pneumophila (L. pneumophila), a causative agent of severe pneumonia. Upon its transmission into the lung, L. pneumophila primarily infects and replicates within macrophages. Here, we analyzed the influence of L. pneumophila OMVs on macrophages. To this end, differentiated THP-1 cells were incubated with increasing doses of Legionella OMVs, leading to a TLR2-dependent classical activation of macrophages with the release of pro-inflammatory cytokines. Inhibition of TLR2 and NF-κB signaling reduced the induction of pro-inflammatory cytokines. Furthermore, treatment of THP-1 cells with OMVs prior to infection reduced replication of L. pneumophila in THP-1 cells. Blocking of TLR2 activation or heat denaturation of OMVs restored bacterial replication in the first 24 h of infection. With prolonged infection-time, OMV pre-treated macrophages became more permissive for bacterial replication than untreated cells and showed increased numbers of Legionella-containing vacuoles and reduced pro-inflammatory cytokine induction. Additionally, miRNA-146a was found to be transcriptionally induced by OMVs and to facilitate bacterial replication. Accordingly, IRAK-1, one of miRNA-146a's targets, showed prolonged activation-dependent degradation, which rendered THP-1 cells more permissive for Legionella replication. In conclusion, L. pneumophila OMVs are initially potent pro-inflammatory stimulators of macrophages, acting via TLR2, IRAK-1, and NF-κB, while at later time points, OMVs facilitate L. pneumophila replication by miR-146a-dependent IRAK-1 suppression. OMVs might thereby promote spreading of L. pneumophila in the host.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Legionella pneumophila Outer Membrane Vesicles: Isolation and Analysis of Their Pro-inflammatory Potential on Macrophages.J Vis Exp. 2017 Feb 22;(120):55146. doi: 10.3791/55146. J Vis Exp. 2017. PMID: 28287548 Free PMC article.
-
Legionella pneumophila infection activates bystander cells differentially by bacterial and host cell vesicles.Sci Rep. 2017 Jul 24;7(1):6301. doi: 10.1038/s41598-017-06443-1. Sci Rep. 2017. PMID: 28740179 Free PMC article.
-
Legionella pneumophila infection-mediated regulation of RICTOR via miR-218 in U937 macrophage cells.Biochem Biophys Res Commun. 2019 Jan 8;508(2):608-613. doi: 10.1016/j.bbrc.2018.11.093. Epub 2018 Dec 1. Biochem Biophys Res Commun. 2019. PMID: 30509489
-
Phosphoinositides and the Fate of Legionella in Phagocytes.Front Immunol. 2020 Jan 30;11:25. doi: 10.3389/fimmu.2020.00025. eCollection 2020. Front Immunol. 2020. PMID: 32117224 Free PMC article. Review.
-
From amoeba to macrophages: exploring the molecular mechanisms of Legionella pneumophila infection in both hosts.Curr Top Microbiol Immunol. 2013;376:1-34. doi: 10.1007/82_2013_351. Curr Top Microbiol Immunol. 2013. PMID: 23949285 Review.
Cited by
-
Extracellular vesicle-derived miRNA as a novel regulatory system for bi-directional communication in gut-brain-microbiota axis.J Transl Med. 2021 May 11;19(1):202. doi: 10.1186/s12967-021-02861-y. J Transl Med. 2021. PMID: 33975607 Free PMC article. Review.
-
Bacterial Outer Membrane Vesicles Promote Lung Inflammatory Responses and Macrophage Activation via Multi-Signaling Pathways.Biomedicines. 2023 Feb 15;11(2):568. doi: 10.3390/biomedicines11020568. Biomedicines. 2023. PMID: 36831104 Free PMC article.
-
Outer membrane vesicles from Neisseria gonorrhoeae target PorB to mitochondria and induce apoptosis.PLoS Pathog. 2018 Mar 30;14(3):e1006945. doi: 10.1371/journal.ppat.1006945. eCollection 2018 Mar. PLoS Pathog. 2018. PMID: 29601598 Free PMC article.
-
Legionella pneumophila Outer Membrane Vesicles: Isolation and Analysis of Their Pro-inflammatory Potential on Macrophages.J Vis Exp. 2017 Feb 22;(120):55146. doi: 10.3791/55146. J Vis Exp. 2017. PMID: 28287548 Free PMC article.
-
Type II Secretion Substrates of Legionella pneumophila Translocate Out of the Pathogen-Occupied Vacuole via a Semipermeable Membrane.mBio. 2017 Jun 20;8(3):e00870-17. doi: 10.1128/mBio.00870-17. mBio. 2017. PMID: 28634242 Free PMC article.
References
-
- Cambronne E. D. and Roy C. R.. 2006. Recognition and delivery of effector proteins into eukaryotic cells by bacterial secretion systems. Traffic (Copenhagen, Denmark).7(8):929–939. - PubMed
-
- Kuehn M. J. and Kesty N. C.. 2005. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes & development.19(22):2645–2655. - PubMed
-
- Ellis T. N., Leiman S. A. and Kuehn M. J.. 2010. Naturally produced outer membrane vesicles from Pseudomonas aeruginosa elicit a potent innate immune response via combined sensing of both lipopolysaccharide and protein components. Infection and immunity.78(9):3822–3831. 10.1128/IAI.00433-10 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

