Sox9 confers stemness properties in hepatocellular carcinoma through Frizzled-7 mediated Wnt/β-catenin signaling
- PMID: 27105493
- PMCID: PMC5045402
- DOI: 10.18632/oncotarget.8835
Sox9 confers stemness properties in hepatocellular carcinoma through Frizzled-7 mediated Wnt/β-catenin signaling
Abstract
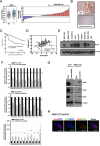
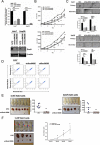
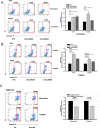
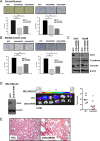
Sox9, an SRY-related HMG box transcription factor, is a progenitor/precursor cell marker of the liver expressed during embryogenesis and following liver injury. In this study, we investigated the role of Sox9 and its molecular mechanism with reference to stemness properties in hepatocellular carcinoma (HCC). Here, we observed upregulation of Sox9 in human HCC tissues compared with the non-tumorous liver counterparts (p < 0.001). Upregulation of Sox9 transcript level was associated with poorer tumor cell differentiation (p = 0.003), venous invasion (p = 0.026), advanced tumor stage (p = 0.044) and shorter overall survival (p = 0.042). Transcript levels of Sox9 and CD24 were positively correlated. Silencing of Sox9 in HCC cells inhibited in vitro cell proliferation and tumorsphere formation, sensitized HCC cells to chemotherapeutic agents, and suppressed in vivo tumorigenicity. In addition, knockdown of Sox9 suppressed HCC cell migration, invasion, and in vivo lung metastasis. Further studies showed that Sox9 endowed stemness features through activation of Wnt/β-catenin signaling, which was confirmed by the partial rescue effect on tumorigenicity and self-renewal upon transfection of active β-catenin in Sox9 knockdown cells. By ChIP and luciferase promoter assays, Frizzled-7 was identified to be the direct transcriptional target of Sox9. In conclusion, Sox9 confers stemness properties of HCC through Frizzled-7 mediated Wnt/β-catenin pathway.
Keywords: Sox9; liver cancer; tumor-initiating cells.
Conflict of interest statement
Nothing to declare.
Figures

Similar articles
-
Cripto-1 contributes to stemness in hepatocellular carcinoma by stabilizing Dishevelled-3 and activating Wnt/β-catenin pathway.Cell Death Differ. 2018 Aug;25(8):1426-1441. doi: 10.1038/s41418-018-0059-x. Epub 2018 Feb 14. Cell Death Differ. 2018. PMID: 29445127 Free PMC article.
-
MicroRNA-452 promotes stem-like cells of hepatocellular carcinoma by inhibiting Sox7 involving Wnt/β-catenin signaling pathway.Oncotarget. 2016 May 10;7(19):28000-12. doi: 10.18632/oncotarget.8584. Oncotarget. 2016. PMID: 27058905 Free PMC article.
-
A SOX9-AS1/miR-5590-3p/SOX9 positive feedback loop drives tumor growth and metastasis in hepatocellular carcinoma through the Wnt/β-catenin pathway.Mol Oncol. 2019 Oct;13(10):2194-2210. doi: 10.1002/1878-0261.12560. Epub 2019 Aug 31. Mol Oncol. 2019. PMID: 31402556 Free PMC article.
-
Integrative analysis of aberrant Wnt signaling in hepatitis B virus-related hepatocellular carcinoma.World J Gastroenterol. 2015 May 28;21(20):6317-28. doi: 10.3748/wjg.v21.i20.6317. World J Gastroenterol. 2015. PMID: 26034368 Free PMC article. Review.
-
Deregulation of Frizzled Receptors in Hepatocellular Carcinoma.Int J Mol Sci. 2018 Jan 21;19(1):313. doi: 10.3390/ijms19010313. Int J Mol Sci. 2018. PMID: 29361730 Free PMC article. Review.
Cited by
-
Transformation of SOX9+ cells by Pten deletion synergizes with steatotic liver injury to drive development of hepatocellular and cholangiocarcinoma.Sci Rep. 2021 Jun 3;11(1):11823. doi: 10.1038/s41598-021-90958-1. Sci Rep. 2021. PMID: 34083580 Free PMC article.
-
Upregulation of GNL3 expression promotes colon cancer cell proliferation, migration, invasion and epithelial-mesenchymal transition via the Wnt/β-catenin signaling pathway.Oncol Rep. 2017 Oct;38(4):2023-2032. doi: 10.3892/or.2017.5923. Epub 2017 Aug 25. Oncol Rep. 2017. PMID: 28849076 Free PMC article.
-
Small bowel carcinomas in celiac or Crohn's disease: distinctive histophenotypic, molecular and histogenetic patterns.Mod Pathol. 2017 Oct;30(10):1453-1466. doi: 10.1038/modpathol.2017.40. Epub 2017 Jun 30. Mod Pathol. 2017. PMID: 28664941
-
EGR1 promotes stemness and predicts a poor outcome of uterine cervical cancer by inducing SOX9 expression.Genes Genomics. 2021 May;43(5):459-470. doi: 10.1007/s13258-021-01064-5. Epub 2021 Mar 9. Genes Genomics. 2021. PMID: 33687657
-
Advance of SOX Transcription Factors in Hepatocellular Carcinoma: From Role, Tumor Immune Relevance to Targeted Therapy.Cancers (Basel). 2022 Feb 24;14(5):1165. doi: 10.3390/cancers14051165. Cancers (Basel). 2022. PMID: 35267473 Free PMC article. Review.
References
-
- Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, Kuhara T, Hosokawa S, Elbahrawy A, Soeda T, Koizumi M, Masui T, Kawaguchi M, Takaori K, et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet. 2011;43:34–41. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

