Inflammasome-independent role of NLRP12 in suppressing colonic inflammation regulated by Blimp-1
- PMID: 27105524
- PMCID: PMC5058702
- DOI: 10.18632/oncotarget.8872
Inflammasome-independent role of NLRP12 in suppressing colonic inflammation regulated by Blimp-1
Abstract
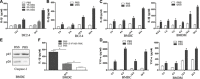
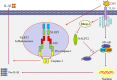
NLRP12 is a member of the Nod-like receptor (NLR). Previous studies have reported enhanced colitis-associated inflammatory responses in NLRP12-deficient mice. In this study, we sought to investigate the role of NLRP12 in DSS-stimulated proinflammatory response in dendritic cells and mice colitis, and the molecular mechanisms involved in the development of the inflammation. Our results showed that down-regulation of NLRP12 is required for DSS-induced release of proinflammatory cytokines IL-1β and TNF-α; that PR domain zinc finger protein 1 (also known as Blimp-1) induces NLRP12 down-regulation during DSS-induced proinflammatory response and colitis; and that TLR4 is implicated in the up-regulation of Blimp-1 that led to the down-regulation of NLRP12 expression in DSS-induced colitis. Taken together, the results suggest that the TLR4-Blimp-1 axis promotes DSS induced experimental colitis through the down-regulation of NLRP12.
Keywords: Blimp-1; NLRP12; TLR4; colitis; inflammation.
Conflict of interest statement
The authors declare no financial or commercial conflicts of interest.
Figures



References
-
- Ishiguro Y. Mucosal proinflammatory cytokine production correlates with endoscopic activity of ulcerative colitis. J Gastroenterol. 1999;34:66–74. - PubMed
-
- Sartor RB. Cytokines in intestinal inflammation: pathophysiological and clinical considerations. Gastroenterol. 1994;106:533–539. - PubMed
-
- Xavier R, Podolsky D. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. - PubMed
-
- Goyette P, Labbé C, Trinh TT, Xavier RJ, Rioux JD. Molecular pathogenesis of inflammatory bowel disease: genotypes, phenotypes and personalized medicine. Ann Med. 2007;39:177–199. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

