Gamma-Aminobutyric Acidergic Projections From the Dorsal Raphe to the Nucleus Accumbens Are Regulated by Neuromedin U
- PMID: 27105831
- PMCID: PMC5016225
- DOI: 10.1016/j.biopsych.2016.02.031
Gamma-Aminobutyric Acidergic Projections From the Dorsal Raphe to the Nucleus Accumbens Are Regulated by Neuromedin U
Abstract
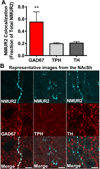
Background: Neuromedin U (NMU) is a neuropeptide enriched in the nucleus accumbens shell (NAcSh), a brain region associated with reward. While NMU and its receptor, NMU receptor 2 (NMUR2), have been studied for the ability to regulate food reward, NMU has not been studied in the context of drugs of abuse (e.g., cocaine). Furthermore, the neuroanatomical pathways that express NMUR2 and its ultrastructural localization are unknown.
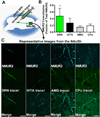
Methods: Immunohistochemistry was used to determine the synaptic localization of NMUR2 in the NAcSh and characterize which neurons express this receptor (n = 17). The functional outcome of NMU on NMUR2 was examined using microdialysis (n = 16). The behavioral effects of NMU microinjection directly to the NAcSh were investigated using cocaine-evoked locomotion (n = 93). The specific effects of NMUR2 knockdown on cocaine-evoked locomotion were evaluated using viral-mediated RNA interference (n = 40).
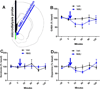
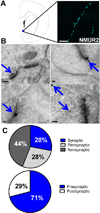
Results: NMUR2 is localized to presynaptic gamma-aminobutyric acidergic nerve terminals in the NAcSh originating from the dorsal raphe nucleus. Furthermore, NMU microinjection to the NAcSh decreased local gamma-aminobutyric acid concentrations. Next, we evaluated the effects of NMU microinjection on behavioral sensitization to cocaine. When repeatedly administered throughout the sensitization regimen, NMU attenuated cocaine-evoked hyperactivity. Additionally, small hairpin RNA-mediated knockdown of presynaptic NMUR2 in the NAcSh using a retrograde viral vector potentiated cocaine sensitization.
Conclusions: Together, these data reveal that NMUR2 modulates a novel gamma-aminobutyric acidergic pathway from the dorsal raphe nucleus to the NAcSh to influence behavioral responses to cocaine.
Keywords: AAV6; Cocaine; GABA; Neuropeptide; Nonserotonergic; RNAi.
Copyright © 2016 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors report no biomedical financial interests or potential conflicts of interest.
Figures
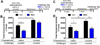



References
-
- Brighton PJ, Szekeres PG, Wise A, Willars GB. Signaling and ligand binding by recombinant neuromedin U receptors: evidence for dual coupling to Galphaq/11 and Galphai and an irreversible ligand-receptor interaction. Mol Pharmacol. 2004;66:1544–1556. - PubMed
-
- Brighton PJ, Szekeres PG, Willars GB. Neuromedin U and its receptors: structure, function, and physiological roles. Pharmacol Rev. 2004;56:231–248. - PubMed
-
- Gartlon J, Szekeres P, Pullen M, Sarau HM, Aiyar N, Shabon U, et al. Localisation of NMU1R and NMU2R in human and rat central nervous system and effects of neuromedin-U following central administration in rats. Psychopharmacology (Berl) 2004;177:1–14. - PubMed
-
- Guan XM, Yu H, Jiang Q, Van Der Ploeg LH, Liu Q. Distribution of neuromedin U receptor subtype 2 mRNA in the rat brain. Brain Res Gene Expr Patterns. 2001;1:1–4. - PubMed
-
- Howard AD, Wang R, Pong SS, Mellin TN, Strack A, Guan XM, et al. Identification of receptors for neuromedin U and its role in feeding. Nature. 2000;406:70–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

