Establishment of a promoter-based chromatin architecture on recently replicated DNA can accommodate variable inter-nucleosome spacing
- PMID: 27106059
- PMCID: PMC5009725
- DOI: 10.1093/nar/gkw331
Establishment of a promoter-based chromatin architecture on recently replicated DNA can accommodate variable inter-nucleosome spacing
Abstract
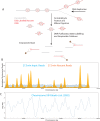
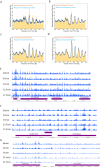
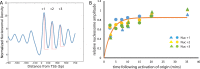
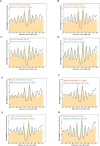
Nucleosomes, the fundamental subunits of eukaryotic chromatin, are organized with respect to transcriptional start sites. A major challenge to the persistence of this organization is the disassembly of nucleosomes during DNA replication. Here, we use complimentary approaches to map the locations of nucleosomes on recently replicated DNA. We find that nucleosomes are substantially realigned with promoters during the minutes following DNA replication. As a result, the nucleosomal landscape is largely re-established before newly replicated chromosomes are partitioned into daughter cells and can serve as a platform for the re-establishment of gene expression programmes. When the supply of histones is disrupted through mutation of the chaperone Caf1, a promoter-based architecture is generated, but with increased inter-nucleosomal spacing. This indicates that the chromatin remodelling enzymes responsible for spacing nucleosomes are capable of organizing nucleosomes with a range of different linker DNA lengths.
© The Author(s) 2016. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

