RIG-I ligand enhances the immunogenicity of recombinant H7HA protein
- PMID: 27106062
- PMCID: PMC5240151
- DOI: 10.1016/j.cellimm.2016.04.004
RIG-I ligand enhances the immunogenicity of recombinant H7HA protein
Abstract
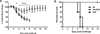
Avian H7N9 influenza virus infection with fatal outcomes continues to pose a pandemic threat and highly immunogenic vaccines are urgently needed. In this report we show that baculovirus-derived recombinant H7 hemagglutinin protein, when delivered with RIG-I ligand, induced enhanced antibody and T cell responses and conferred protection against lethal challenge with a homologous H7N9 virus. These findings indicate the potential utility of RIG-I ligands as vaccine adjuvants to increase the immunogenicity of recombinant H7 hemagglutinin.
Keywords: Antibody responses; Cell-mediated immunity; H7HA; Influenza; Mouse model; Protective immunity; RIG-I ligand.
Published by Elsevier Inc.
Conflict of interest statement
Potential conflicts of interest: All authors report no potential conflicts.
Figures

References
-
- Hoelscher M, Gangappa S, Zhong W, Jayashankar L, Sambhara S. Vaccines against epidemic and pandemic influenza. Expert Opin Drug Deliv. 2008;5:1139–1157. - PubMed
-
- U.S.F.a.D. Administration. FDA approves first adjuvanted vaccine for prevention of H5N1 avian influenza. 2013 Nov
-
- Nohynek H, Jokinen J, Partinen M, Vaarala O, Kirjavainen T, Sundman J, Himanen SL, Hublin C, Julkunen I, Olsen P, Saarenpaa-Heikkila O, Kilpi T. AS03 adjuvanted AH1N1 vaccine associated with an abrupt increase in the incidence of childhood narcolepsy in Finland. PLoS One. 2012;7:e33536. - PMC - PubMed
-
- Dauvilliers Y, Arnulf I, Lecendreux M, Monaca Charley C, Franco P, Drouot X, d'Ortho MP, Launois S, Lignot S, Bourgin P, Nogues B, Rey M, Bayard S, Scholz S, Lavault S, Tubert-Bitter P, Saussier C, Pariente A, Narcoflu VFsg. Increased risk of narcolepsy in children and adults after pandemic H1N1 vaccination in France. Brain. 2013;136:2486–2496. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

