A missense mutation in ASRGL1 is involved in causing autosomal recessive retinal degeneration
- PMID: 27106100
- PMCID: PMC6086560
- DOI: 10.1093/hmg/ddw113
A missense mutation in ASRGL1 is involved in causing autosomal recessive retinal degeneration
Abstract
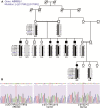
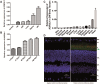
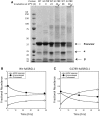
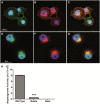
Inherited retinal dystrophies are a group of genetically heterogeneous conditions with broad phenotypic heterogeneity. We analyzed a large five-generation pedigree with early-onset recessive retinal degeneration to identify the causative mutation. Linkage analysis and homozygosity mapping combined with exome sequencing were carried out to map the disease locus and identify the p.G178R mutation in the asparaginase like-1 gene (ASRGL1), segregating with the retinal dystrophy phenotype in the study pedigree. ASRGL1 encodes an enzyme that catalyzes the hydrolysis of L-asparagine and isoaspartyl-peptides. Studies on the ASRGL1 expressed in Escherichia coli and transiently transfected mammalian cells indicated that the p.G178R mutation impairs the autocatalytic processing of this enzyme resulting in the loss of functional ASRGL1 and leaving the inactive precursor protein as a destabilized and aggregation-prone protein. A zebrafish model overexpressing the mutant hASRGL1 developed retinal abnormalities and loss of cone photoreceptors. Our studies suggest that the p.G178R mutation in ASRGL1 leads to photoreceptor degeneration resulting in progressive vision loss.
© The Author 2016. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


References
-
- Heckenlively J.R. (1988) Retinitis Pigmentosa. J.B. Lippincott Company, Philadelphia.
-
- Thompson D.A., Ali R.R., Banin E., Branham K.E., Flannery J.G., Gamm D.M., Hauswirth W.W., Heckenlively J.R., Iannaccone A., Jayasundera K.T, et al. (2015) Advancing therapeutic strategies for inherited retinal degeneration: recommendations from the Monaciano Symposium. Investigative Ophthalmology and Visual Science, 56, 918–931. - PMC - PubMed
-
- Roosing S., Thiadens A.A., Hoyng C.B., Klaver C.C., den Hollander A.I., Cremers F.P. (2014) Causes and consequences of inherited cone disorders. Prog. Retin. Eye Res., 42, 1–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

