The vitronectin RGD motif regulates TGF-β-induced alveolar epithelial cell apoptosis
- PMID: 27106291
- PMCID: PMC4935469
- DOI: 10.1152/ajplung.00424.2015
The vitronectin RGD motif regulates TGF-β-induced alveolar epithelial cell apoptosis
Abstract
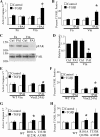
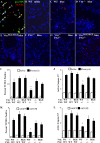
Transforming growth factor-β (TGF-β) is a critical driver of acute lung injury and fibrosis. Injury leads to activation of TGF-β, which regulates changes in the cellular and matrix makeup of the lung during the repair and fibrosis phase. TGF-β can also initiate alveolar epithelial cell (AEC) apoptosis. Injury leads to destruction of the laminin-rich basement membrane, which is replaced by a provisional matrix composed of arginine-glycine-aspartate (RGD) motif-containing plasma matrix proteins, including vitronectin and fibronectin. To determine the role of specific matrix proteins on TGF-β-induced apoptosis, we studied primary AECs cultured on different matrix conditions and utilized mice with deletion of vitronectin (Vtn(-/-)) or mice in which the vitronectin RGD motif is mutated to nonintegrin-binding arginine-glycine-glutamate (RGE) (Vtn(RGE/RGE)). We found that AECs cultured on fibronectin and vitronectin or in wild-type mouse serum are resistant to TGF-β-induced apoptosis. In contrast, AECs cultured on laminin or in serum from Vtn(-/-) or Vtn(RGE/RGE) mice undergo robust TGF-β-induced apoptosis. Plasminogen activator inhibitor-1 (PAI-1) sensitizes AECs to greater apoptosis by disrupting AEC engagement to vitronectin. Inhibition of integrin-associated signaling proteins augments AEC apoptosis. Mice with transgenic deletion of PAI-1 have less apoptosis after bleomycin, but deletion of vitronectin or disruption of the vitronectin RGD motif reverses this protection, suggesting that the proapoptotic function of PAI-1 is mediated through vitronectin inhibition. Collectively, these data suggest that integrin-matrix signaling is an important regulator of TGF-β-mediated AEC apoptosis and that PAI-1 functions as a natural regulator of this interaction.
Keywords: apoptosis; epithelial; fibrosis; lung; matrix.
Copyright © 2016 the American Physiological Society.
Figures

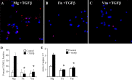
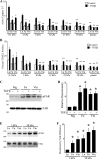
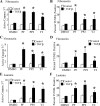
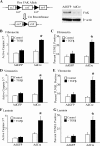



References
-
- Akiyama SK. Purification of vitronectin. Curr Protoc Cell Biol 60: 16, 2013. - PubMed
-
- Bale MD, Wohlfahrt LA, Mosher DF, Tomasini B, Sutton RC. Identification of vitronectin as a major plasma protein adsorbed on polymer surfaces of different copolymer composition. Blood 74: 2698–2706, 1989. - PubMed
-
- Barnes DW, Silnutzer J. Isolation of human serum spreading factor. J Biol Chem 258: 12548–12552, 1983. - PubMed
-
- Beausejour M, Noel D, Thibodeau S, Bouchard V, Harnois C, Beaulieu JF, Demers MJ, Vachon PH. Integrin/Fak/Src-mediated regulation of cell survival and anoikis in human intestinal epithelial crypt cells: selective engagement and roles of PI3-K isoform complexes. Apoptosis 17: 566–578, 2012. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

