Enteric glia: the most alimentary of all glia
- PMID: 27106597
- PMCID: PMC5233670
- DOI: 10.1113/JP271021
Enteric glia: the most alimentary of all glia
Abstract
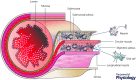
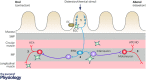
Glia (from Greek γλοία meaning 'glue') pertains to non-neuronal cells in the central (CNS) and peripheral nervous system (PNS) that nourish neurons and maintain homeostasis. In addition, glia are now increasingly appreciated as active regulators of numerous physiological processes initially considered exclusively under neuronal regulation. For instance, enteric glia, a collection of glial cells residing within the walls of the intestinal tract, regulate intestinal motility, a well-characterized reflex controlled by enteric neurons. Enteric glia also interact with various non-neuronal cell types in the gut wall such as enterocytes, enteroendocrine and immune cells and are therefore emerging as important local regulators of diverse gut functions. The intricate molecular mechanisms that govern glia-mediated regulation are beginning to be discovered, but much remains unknown about the functions of enteric glia in health and disease. Here we present a current view of the enteric glia and their regulatory roles in gastrointestinal (GI) (patho)physiology; from GI motility and epithelial barrier function to enteric neuroinflammation.
© 2016 The Authors. The Journal of Physiology © 2016 The Physiological Society.
Figures





References
-
- Abreu MT (2010). Toll‐like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol 10, 131–144. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

