Recombinant versus urinary human chorionic gonadotrophin for final oocyte maturation triggering in IVF and ICSI cycles
- PMID: 27106604
- PMCID: PMC7133782
- DOI: 10.1002/14651858.CD003719.pub4
Recombinant versus urinary human chorionic gonadotrophin for final oocyte maturation triggering in IVF and ICSI cycles
Abstract
Background: For the last few decades urinary human chorionic gonadotrophin (uhCG) has been used to trigger final oocyte maturation in cycles of in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI). Recombinant technology has allowed the production of two drugs, recombinant human chorionic gonadotrophin (rhCG) and recombinant luteinising hormone (rLH), that can be used for the same purpose, to mimic the endogenous luteinising hormone (LH) surge. This allows commercial manufacturers to adjust production according to market requirements and to remove all urinary contaminants, facilitating the safe subcutaneous administration of a compound with less batch-to-batch variation. However, prior to a change in practice, it is necessary to compare the effectiveness of the recombinant drugs to the currently used urinary human chorionic gonadotrophin (uhCG).
Objectives: To assess the effects of subcutaneous rhCG and high dose rLH versus uhCG for inducing final oocyte maturation in subfertile women undergoing IVF and ICSI cycles.
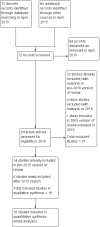
Search methods: We searched the Cochrane Menstrual Disorders and Subfertility Group Trials Register (April 2015), the Cochrane Central Register of Controlled Trials (CENTRAL) (2015, Issue 3), MEDLINE (1946 to April 2015), EMBASE (1980 to April 2015) and PsycINFO (1806 to April 2015) as well as trial registers at ClinicalTrials.gov on 13 May 2015 and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) search portal on 14 May 2015.
Selection criteria: Two review authors independently scanned titles and abstracts and selected those that appeared relevant for collection of the full paper. We included randomised controlled trials comparing rhCG and rLH with urinary hCG for final oocyte maturation triggering in IVF and ICSI cycles for treatment of infertility in normogonadotropic women.
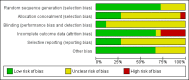
Data collection and analysis: Two authors independently performed assessment for inclusion or exclusion, quality assessment and data extraction. We discussed any discrepancies in the presence of a third author to reach a consensus. The primary review outcomes were ongoing pregnancy/live birth and incidence of ovarian hyperstimulation syndrome (OHSS). Clinical pregnancy, miscarriage rate, number of oocytes retrieved and adverse events were secondary outcomes. We combined data to calculate pooled odds ratios (ORs) and 95% confidence intervals (CIs) and assessed statistical heterogeneity using the I(2) statistic. We evaluated the overall quality of the evidence for the main comparisons using GRADE methods.
Main results: We included 18 RCTs involving 2952 participants; 15 compared rhCG with uhCG, and 3 compared rhLH with uhCG. The evidence for different comparisons ranged from very low to high quality: limitations were poor reporting of study methods and imprecision. Pharmaceutical companies funded 9 of the 18 studies, and 5 studies did not clearly report funding source. Ongoing pregnancy/live birthThere was no conclusive evidence of a difference between rhCG and uhCG (OR 1.15, 95% CI 0.89 to 1.49; 7 RCTs, N = 1136, I(2) = 0%, moderate quality evidence) or between rhLH and uhCG (OR 0.95, 95% CI 0.51 to 1.78, 2 RCTs, N = 289, I(2) = 0%, very low quality evidence) for ongoing pregnancy/live birth rates. OHSS There was no evidence of a difference between rhCG and uhCG in the incidence of OHSS: moderate to severe OHSS (OR 1.76, 95% CI 0.37 to 8.45; 3 RCTs, N = 417, I(2) = 0%, low quality evidence), moderate OHSS (OR 0.78, 95% CI 0.27 to 2.27; 1 RCT, N = 243, I(2) = 0%, low quality evidence), mild to moderate OHSS (OR 1.00, 95% CI 0.42 to 2.38; 2 RCTs, N = 320, I(2) = 0%, low quality evidence) or undefined OHSS (OR 1.18, 95% CI 0.50 to 2.78; 3 RCTs, N = 495, I(2) = 0%, low quality evidence). Likewise, there was no evidence of a difference between rhLH and uhCG in OHSS rates for moderate OHSS (OR 0.82, 95% CI 0.39 to 1.69, 2 RCTs, N = 280, I(2) = 5%, very low quality evidence). Other adverse events There was no evidence of a difference in miscarriage rates between rhCG and uhCG (OR 0.72, 95% CI 0.41 to 1.25; 8 RCTs, N = 1196, I(2) = 0%, low quality evidence) or between rhLH and uhCG (OR 0.95, 95% CI 0.38 to 2.40; 2 RCTs, N = 289, I(2) = 0%, very low quality evidence). For other adverse effects (most commonly injection-site reactions) rhCG was associated with a lower number of adverse events than uhCG (OR 0.52, 95% CI 0.35 to 0.76; 5 RCTS, N = 561; I(2) = 67%, moderate quality evidence). However, when we used a random-effects model due to substantial statistical heterogeneity, there was no evidence of a difference between the groups (OR 0.56, 95% CI 0.27 to 1.13). Only one study comparing rLH and uhCG reported other adverse events, and it was impossible to draw conclusions.
Authors' conclusions: We conclude that there is no evidence of a difference between rhCG or rhLH and uhCG for live birth or ongoing pregnancy rates or rates of OHSS.
Conflict of interest statement
Previous author Hesham Al‐Inany contributed to one of the trials included in this review (Study 21447).
No current author has any interest to declare.
Figures



















Update of
-
Recombinant versus urinary human chorionic gonadotrophin for final oocyte maturation triggering in IVF and ICSI cycles.Cochrane Database Syst Rev. 2011 Apr 13;(4):CD003719. doi: 10.1002/14651858.CD003719.pub3. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2016 Apr 23;4:CD003719. doi: 10.1002/14651858.CD003719.pub4. PMID: 21491386 Updated.
References
References to studies included in this review
Abdelmassih 2005 {published data only}
-
- Abdelmassih V, Oliveira FG, Goncalves SP, Varella AD, Diamond MP, Abdelmassih R. A prospective randomized and blinded comparison between urinary and recombinant HCG for oocyte maturation in IVF cycles. [Abstract]. Fertility and sterility 2003;80(Suppl 3):69‐70.
-
- Abdelmassih V, Oliveira FG, Goncalves SP, Varella AD, Diamond MP, Abdelmassih R. A prospective, randomized and blinded comparison between 10,000 IU urinary and 250 µg recombinant human chorionic gonadotropin for oocyte maturation in in vitro fertilization cycles. Journal of Assisted Reproduction and Genetics 2005;22(4):149‐53. - PMC - PubMed
Bellavia 2013 {published data only}
-
- Bellavia M, Geyter C, Streuli I, Ibecheole V, Birkhauser MH, Cometti BPS, Ziegler D. Randomized controlled trial comparing highly purified (HP‐hCG) and recombinant hCG (r‐hCG) for triggering ovulation in ART. Gynecological Endocrinology 2013;29(2):93‐7. - PubMed
-
- NCT00335569. Clinical tolerance and equivalence of hCG‐IBSA vs recombinant human chorionic gonadotrophin in women undergoing in vitro fertilisation. https://clinicaltrials.gov/ct2/show/NCT00335569 (accessed 20 May 2015).
Borges 2004 {published data only}
-
- Borges E Jr, Iaconelli A Jr, Bonetti TC, Maldonado LG, Bibancos M, Busato WC. The effectiveness of recombinant versus urinary human chorionic gonadotropin in IVF. Fertility and Sterility 2004;82(Suppl 2):35.
Chang 2001 {published data only}
-
- Chang P, Kenley S, Burns T, Denton G, Currie K, DeVane G, et al. Recombinant human chorionic gonadotropin (rhCG) in assisted reproductive technology: results of a clinical trial comparing two doses of rhCG (Ovidrel) to urinary hCG (Profasi) for induction of final follicular maturation in in vitro fertilization‐embryo transfer. Fertility and Sterility 2001;76(1):67‐74. - PubMed
Driscoll 2000 {published data only}
-
- Driscoll GL, Tyler JP, Hangan JT, Fisher PR, Birdsall MA, Knight DC. A prospective, randomized, controlled, double‐blind, double‐dummy comparison of recombinant and urinary HCG for inducing oocyte maturation and follicular luteinization in ovarian stimulation. Human Reproduction 2000;15(6):1305‐10. - PubMed
Eftekhar 2012 {published data only}
ERHCG Group 2000 {published data only}
-
- Mills J, Maislisch L, Warne D. Induction of final follicular maturation and early luteinization in women undergoing ovulation induction for assisted reproduction: recombinant human chorionic gonadotrophin (Ovidrel®) versus urinary HCG (Profasi®). Human Reproduction 2000;15(A1):46. - PubMed
-
- The European Recombinant Human Chorionic Gonadotrophin Study Group. Induction of final follicular maturation and early luteinization in women undergoing ovulation induction for assisted reproduction treatment ‐ recombinant HCG versus urinary HCG. Human Reproduction 2000;15(7):1446‐51. - PubMed
-
- Warne D, Hughes JN, Durnerin IC, Decosterd G, Loumaye E. The efficacy and safety of recombinant‐ versus urinary‐human chorionic gonadotrophin in ovulation induction: results of a double‐blind clinical study. Abstract of the annual meeting of ESHRE 2000;15 Suppl(A1):87.
ERLH Group 2001 {published data only}
-
- The European Recombinant LH Study Group. Recombinant human luteinizing hormone is as effective as, but safer than, urinary human chorionic gonadotropin in inducing final follicular maturation and ovulation in in vitro fertilization procedures: results of a multicenter double‐blind study. Journal of Clinical Endocrinology and Metabolism 2001;86(6):2607‐18. - PubMed
Farrag 2008 {published data only}
Goswami 2007 {published data only}
-
- Goswami K, Ghosh S, Chattopadhyay R, Sharma S, Chakravarty BN. Can recombinant human chorionic gonadotropin (r‐hCG) lower the incidence of ovarian hyperstimulation syndrome (OHSS) in PCO?. Fertility and Sterility 2007;88(Suppl 1):150.
Jie 2005 {published data only}
-
- Jie Q, Guijin Z, Guoning H. A randomized study of recombinant versus urinary human chorionic gonadotrophin in Chinese women undergoing assisted reproductive technology cycles. Fertility and Sterility 2005;84(Suppl 1):301.
-
- NCT01081756. A Study Using Recombinant Human Chorionic Gonadotrophin (rhCG, Ovidrel®) in the Induction of Final Follicle Maturation and Early Luteinization in Chinese Women Undergoing in Vitro Fertilization and Embryo Transfer (IVF/ET). https://clinicaltrials.gov/ct2/show/NCT01081756 (accessed 20 May 2015).
Kovacs 2008 {published data only}
-
- Kovacs P, Kovats T, Bernard A. Comparison of serum and follicular fluid hormone levels with recombinant and urinary human chorionic gonadotropin during in vitro fertilization. Fertility and Sterility 2008;90(6):2133‐7. - PubMed
Madani 2013 {published data only}
-
- Madani T, Yeganeh LM, Ezabadi Z, Hasani F, Chehrazi M. Comparing the efficacy of urinary and recombinant hCG on oocyte/follicle ratio to trigger ovulation in women undergoing intracytoplasmic sperm injection cycles: a randomized controlled trial. Journal of Assisted Reproduction and Genetics 2013;30(2):239‐45. - PMC - PubMed
-
- NCT01507376. Comparing the efficacy of urinary and recombinant human chorionic gonadotropin (hCG) on oocyte maturity. https://clinicaltrials.gov/ct2/show/NCT01507376 (accessed 20 May 2015).
Manau 2002 {published data only}
-
- Manau D, Fabregues F, Arroyo V, Jimenez W, Vanrell JA, Balasch J. Hemodynamic changes induced by urinary human chorionic gonadotropin and recombinant luteinizing hormone used for inducing final follicular maturation and luteinization. Fertility and Sterility 2002;78(6):1261‐7. - PubMed
Papanikolaou 2010 {published data only}
-
- NCT00953628. Endometrial advancement after Rec or u‐HCG triggering. https://clinicaltrials.gov/ct2/show/NCT00953628 (accessed 20 May 2015).
-
- NCT00954265. Pregnancy rates in IVF after ovulation triggering with recombinant or urinary human chorionic gonadotrophin (HCG). https://clinicaltrials.gov/ct2/show/NCT00954265 (accessed 20 May 2015).
-
- Papanikolaou EG, Fatemi H, Camus M, Kyrou D, Nikos PP, Humaidan P, et al. Higher birth rate after recombinant hCG triggering compared with urinary‐derived hCG in single‐blastocyst IVF antagonist cycles: a randomized controlled trial. Fertility and Sterility 2010;94(7):2902‐4. - PubMed
Schoolcraft 2002 {published data only}
-
- Schoolcraft W, Surrey E, Gardner D, Adams C, Stevens J. Recombinant hCG (Ovidrel®) for ovulation triggering in ART. Fertility and Sterility 2000;77(Suppl 3):20.
Study 21447 {unpublished data only}
-
- [No authors listed]. Study 21447: Luverisis (r‐hLH) compared to u‐hCG. Serono (unpublished data).
Vidal 2005 {published data only}
-
- Vidal C, Zuzuarregui J, Epifanio R, Simon C, Remohi J, Pellicer A. Urinary human chorionic gonadotropin (hCG), intramuscular or subcutaneously administered, yields higher hCG serum and follicular levels than recombinant hCG in egg donors. [Abstract]. Fertility and sterility 2005;84(Suppl 1):296‐7.
References to studies excluded from this review
Abdelmassih 2004 {published data only}
-
- Abdelmassih V, Abdelmassih R. Confirmatory results on efficacy of recombinant human chorionic gonadotropin (r‐hCG) for final follicular maturation in patients undergoing controlled ovarian hyperstimulation (COH) for assisted reproductive technologies (ART). An observational study. [Abstract]. Fertility and sterility 2004;82(Suppl 2):239.
Acosta 2003 {published data only}
-
- Acosta M, Mukherjee T, Grunfeld L, Sandler B, Bergh P, Copperman A. Use of subcutaneous HCG (Ovidrel) for final follicular maturation does not change reproductive outcome. Fertility and Sterility 2003;80(Suppl 3):246‐7.
Adeniya 2004 {published data only}
-
- Adaniya G, Bonaventura L, Colver R, Bopp B, Reuter L. Effect of recombinant versus urinary human chorionic gonadotropin administration on assisted reproductive technology cycles. Fertility and Sterility 2004;81(Suppl 3):276.
Aleyasin 2012 {published data only}
-
- Aleyasin A, Mahdavi A, Agha Hosseini AH, Safdarian L, Fallahi P, Bahmaee F. Comparison of two doses of recombinant human chorionic gonadotropin and urinary human chorionic gonadotropin during intracytoplasmic sperm injection cycles. Human Reproduction 2012;27(Suppl 2):P‐324.
Allamaneni 2005 {published data only}
-
- Allamaneni SS, Pasqualotto EB, Ogliari KS, Fritsch M, Agarwal A, Pasqualotto FF. Recombinant versus urinary hCG for ovulation induction in assisted reproduction. Fertility and Sterility 2005;84(Suppl 1):299.
Antoine 1997 {published data only}
-
- Antoine JM, Loumaye E, Alvarez S, Merviel P, Cornet D, Mandelbaum J. Clinical use of recombinant gonadotropins (FSH, LH, hCG) [L'utilisation en clinique des hormones gonadotropes recombinantes (FSH, LH, hCG)]. Contraception, Fertilite, Sexualite 1997;25(2):141‐6. - PubMed
Balasch 2003 {published data only}
-
- Balasch J, Fabregues F. Pregnancy after administration of high dose recombinant human LH alone to support final stages of follicular maturation in a woman with long‐standing hypogonadotrophic hypogonadism. Reproductive Biomedicine Online 2003;6(4):427‐31. - PubMed
Daya 2002 {published data only}
-
- Daya S, Gunby J. Singleton pregnancy resulting from recombinant versus urinary human chorionic gonadotropin?a meta‐analysis. Fertility and Sterility 2002;78(Suppl 1):147‐8.
Emperaire 1994 {published data only}
-
- Emperaire JC. Therapeutic induction of ovulation: towards the replacement of hCG with LH. Contraception, Fertilite, Sexualite 1994;22(7‐8):459‐67. - PubMed
Forghani 2012 {unpublished data only}
-
- Forghani F. Comparison of urinary and recombinant human chorionic gonadotropin during ovulation induction in intrauterine insemination cycles. International Journal of Fertility and Sterility 2012;6(Suppl 1):109. - PubMed
Gardella 2003 {published data only}
-
- Gardella JR, Lesser CB, Penzias AS, Alper MM. Recombinant human chorionic gonadotropin (R‐HCG) and urinary HCG (U‐HCG) are equally effective with controlled ovarian stimulation in egg donors. Fertility and Sterility 2003;80(Suppl 3):245‐6.
Gill 2004 {published data only}
-
- Gill M, Marchetti J, Martinez J, Rosenwaks Z. Efficacy and ease of use of a new liquid formulation of recombinant human chorionic gonadotropin (r‐hCG) utilized for final follicle maturation in an ovum donation (OD) program. Fertility and Sterility 2004;82(Suppl 2):336.
Hreinsson 2003 {published data only}
-
- Hreinsson J, Rosenlund B, Friden B, Levkov L, Ek I, Suikkari AM, et al. Recombinant LH is equally effective as recombinant hCG in promoting oocyte maturation in a clinical in‐vitro maturation programme: a randomized study. Human Reproduction 2003;18(10):2131‐6. - PubMed
Hugues 2004 {published data only}
-
- Hugues JN. Comparative use of urinary and recombinant human chorionic gonadotropins in women. Treatments in Endocrinology 2004;3:371‐9. - PubMed
IRHCG Group 2001 {published data only}
-
- Hugues JN. Induction of ovulation in World Health Organization group II anovulatory women undergoing follicular stimulation with recombinant human follicle‐stimulating hormone: a comparison of recombinant human chorionic gonadotropin (rhCG) and urinary hCG. Fertility and Sterility 2001;75(6):1111‐8. - PubMed
-
- NCT01735422. A study to evaluate safety and efficacy of recombinant human luteinizing hormone (r‐hLH) compared with urinary human chorionic gonadotrophin (u‐hCG) to trigger ovulation in infertile women. https://clinicaltrials.gov/ct2/show/NCT01735422 (accessed 20 May 2015).
-
- The International Recombinant Human Chorionic Gonadotropin Study Group. Induction of ovulation in World Health Organization group II anovulatory women undergoing follicular stimulation with recombinant human follicle‐stimulating hormone: a comparison of recombinant human chorionic gonadotropin (rhCG) and urinary hCG. Fertility and Sterility 2001;75(6):1111‐8. - PubMed
Kaplan 2020 {published data only}
-
- Kaplan PF, Austin DJ, Keobounnam M, Patton PE. A prospective, randomized trial of subcutaneous versus intramuscular urinary hCG for ovulation induction. Fertility and Sterility 2002;77(3):S12‐S13.
Kim 2014 {published data only}
-
- Kim CH, Ahn JW, You RM, Kim SH, Chae HD, Kang BM. Combined administration of gonadotropin‐releasing hormone agonist with human chorionic gonadotropin for final oocyte maturation in GnRH antagonist cycles for in vitro fertilization. The Journal of Reproductive Medicine 2014;59(1‐2):63‐8. - PubMed
Klipstein 2003 {published data only}
-
- Klipstein S, Alper MM. Recombinant human chorionic gonadotropin (RHCG): a comparison of 250 versus 400 µg doses. Fertility and Sterility 2003;80(Suppl 3):181.
Kolibianakis 2007 {published data only}
-
- Kolibianakis EM, Papanikolaou EG, Tournaye H, Camus M, Steirteghem AC, Devroey P. Triggering final oocyte maturation using different doses of human chorionic gonadotropin: a randomized pilot study in patients with polycystic ovary syndrome treated with gonadotropin‐releasing hormone antagonists and recombinant follicle‐stimulating hormone. Fertility and Sterility 2007;88(55):1382‐8. - PubMed
Krotz 2008 {published data only}
-
- Krotza SP, Bhagavatha B, Hacketta R, Pagidasa K, Carsona SA, Robins J. Comparison of IVF retrieval and clinical outcomes in 744 patients using recombinant versus urinary human chorionic gonadotropins to trigger ovulation. Fertility and Sterility 2008;80(Suppl 1):224‐5.
Littman 2003 {published data only}
-
- Littman ED, Milki AA. The combination of urinary and recombinant HCG improves outcome in patients with decreased oocyte/follicle ratio in previous cycles. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2003;109(1):60‐2. - PubMed
Lorusso 2008 {published data only}
-
- Lorusso F, Palmisano M, Serrat G, Bassi E, Lamanna G, Vacca M, et al. Intrauterine insemination with recombinant or urinary human chorionic gonadotropin: A prospective randomized trial. Gynecological Endocrinology 2008;24(11):644‐8. - PubMed
Ludwig 2003 {published data only}
-
- Ludwig M, Doody KJ, Doody KM. Use of recombinant human chorionic gonadotropin in ovulation induction. Fertility and Sterility 2003;79(5):1051‐9. - PubMed
Mendez 2006 {published data only}
-
- Mendez V, Pinillos M, Rubio M, Gonzalez FS. Intramuscular (IM) vs subcutaneous (SC) human chorionic gonadotropin (hCG) administration to trigger LH peak in controlled ovarian stimulation (COS) cycles for IVF. Fertility and Sterility 2006;86(Suppl 1):154.
Penarrubia 1999 {published data only}
-
- Penarrubia J, Balasch J, Fabregues F, Creus M, Civico S, Vanrell JA. Recurrent empty follicle syndrome successfully treated with recombinant human chorionic gonadotrophin. Human Reproduction 1999;14(7):1703‐6. - PubMed
Pierson 2014 {published data only}
-
- Pierson RA, Olatunbosun OA, Chizen DR, Sundersd H, Loumaye E, Moustier B. Recombinant human luteinizing hormone to trigger ovulation: randomized, controlled, dose‐finding pilot study in ovulation induction. Journal of Reproductive Medicine 2014;59(7‐8):355‐66. - PubMed
Retzloff 2002 {published data only}
-
- Retzloff MG, Jackson KV, Fox JH, Ginsburg ES, Racowsky C. Use of recombinant hCG results in reduced IVF pregnancy rates compared to non‐recombinant u‐hCG. Fertility and Sterility 2002;78(Suppl):44.
Revelli 2006 {published data only}
-
- NCT00415766. Comparison between recombinant versus urinary hCG for ovulation induction. https://clinicaltrials.gov/ct2/show/NCT00415766 (accessed 20 May 2015).
-
- Revelli A, Poso F, Gennerelli G, Moffa F, Grassi G, Massobrio M. Recombinant versus highly‐purified, urinary follicle‐stimulating hormone (r‐FSH vs. HP‐uFSH) in ovulation induction: a prospective, randomized study with cost‐minimization analysis. Reproductive Biology and Endocrinology 2006;4:38. - PMC - PubMed
Ryley 2005 {published data only}
-
- Ryley DA, Imon A, Wald T, Ezcurra D, Alper MM. Comparing the efficacy of recombinant human chorionic gonadotropin (r‐hCG) versus urinary hCG (u‐hCG) in achieving final follicular maturation in patients undergoing assisted reproductive technologies (ART). [Abstract]. Fertility and sterility 2005;84 Suppl(1):309.
Sakhel 2007 {published data only}
-
- Sakhel K, Khedr M, Schwark S, Ashraf M, Fakih MH, Abuzeid M. Comparison between urinary and recombinant hCG during ovulation induction in IUI cycles: a prospective randomized clinical trial. Fertility and Sterility 2004;82(Suppl 2):238. - PubMed
-
- Sakhel K, Khedr M, Schwark S, Ashraf M, Fakih MH, Abuzeid M. Comparison of urinary and recombinant human chorionic gonadotropin during ovulation induction in intrauterine insemination cycles: a prospective randomized clinical trial. Fertility and Sterility 2007;87(6):1357‐62. - PubMed
Sidhmalswamy 2012 {published data only}
-
- Sidhmalswamy GSA, Srinivas MS, Dipika K, Anu K, Rao KA, Mekhala D. Comparing the efficacy of urinary hCG vs recombinant hCG for final maturation of oocyte in GnRH antagonist IVF/ICSI cycle. International Journal of Infertility and Fetal Medicine 2012;3(3):92‐6.
Sneeringer 2008 {published data only}
-
- Sneeringera R, Alpera MM, Ezcurraa D. Comparing the efficacy of recombinant human chorionic gonadotropin versus urinary hCG in achieving final follicular maturation in 9328 patients undergoing assisted reproductive technologies (ART). Fertility and Sterility 2008;90(Suppl 1):233.
Sulit 2006 {published data only}
-
- Sulit MT, Ezcurra D, Rosenwaks Z. Comparing the efficacy of recombinant human chorionic gonadotropin (r‐hCG) versus urinary hCG (u‐hCG) in patients who underwent controlled ovarian hyperstimulation (COH)/IUI cycles. Fertility and Sterility 2006;86(3):92‐3.
Uhler 2006 {published data only}
-
- Uhler ML, Beltsos AN, Grotjan HE, Lederer KJ, Lifchez AS. Age‐matched comparison of recombinant and urinary HCG for final follicular maturation. Reproductive Biomedicine Online 2006;13(3):315‐29. - PubMed
-
- Uhler ML, Beltsos AN, Lederer KJ, Lifchez AS. Comparison of recombinant and urinary human chorionic gonadotropin (hCG) for induction of final follicular maturation in a large IVF program. Fertility and Sterility 2004;82 Suppl 2:238.
Zelinski‐Wooten 1997 {published data only}
-
- Zelinski‐Wooten MB, Hutchison JS, Trinchard‐Lugan I, Hess DL, Wolf DP, Stouffer RL. Initiation of periovulatory events in gonadotrophin‐stimulated macaques with varying doses of recombinant human chorionic gonadotrophin. Human Reproduction 1997;12(9):1877‐85. - PubMed
Zreik 1999 {published data only}
-
- Zreik TG, García‐Velasco JA, Habboosh MS, Olive DL, Arici A. Prospective, randomized, crossover study to evaluate the benefit of human chorionic gonadotropin‐timed versus urinary luteinizing hormone‐timed intrauterine inseminations in clomiphene citrate‐stimulated treatment cycles. Fertility and Sterility 1999;71(6):1070‐4. - PubMed
References to studies awaiting assessment
NCT01081756 {published data only}
-
- NCT01081756. A study using recombinant human chorionic gonadotrophin (rhCG, Ovidrel®) in the induction of final follicle maturation and early luteinization in Chinese women undergoing in vitro fertilization and embryo transfer (IVF/ET). https://clinicaltrials.gov/ct2/show/NCT01081756 (accessed 20 May 2015). [IMP‐25346; NCT01081756 ]
NCT01735422 {published data only}
-
- NCT01735422. A phase II, single center, open, randomized, parallel group, dose finding study to assess the safety and efficacy of recombinant human luteinizing hormone (r‐hLH), compared with urinary human chorionic gonadotrophin (u‐hCG), in inducing ovulation in infertile women undergoing stimulation of follicular development with recombinant human follicle stimulating hormone (Gonal F®). https://clinicaltrials.gov/ct2/show/NCT01735422 (accessed 20 May 2015). [21321; NCT01735422]
References to ongoing studies
ChiCTR‐TRC‐12002072 {published data only}
-
- ChiCTR‐TRC‐12002072. Ovarian response in poor ovarian responder: a randomized controlled trial on the effect of mid‐follicular phase recombinant luteinizing hormone versus low dose urinary human chorionic gonadotrophin supplement on in‐vitro fertilization cycles. http://www.chictr.org.cn/showproj.aspx?proj=7477 (accessed 20 May 2015).
NCT01653743 {published data only}
-
- NCT01653743. Trial to Assess the Clinical Efficacy and Safety of MSJ‐0011 in Inducing Ovulation in Anovulatory or Oligo‐ovulatory Japanese Women. https://clinicaltrials.gov/ct2/show/NCT01653743 (accessed 20 May 2015).
Additional references
Aboulghar 1998
-
- Aboulghar MA, Mansour RT, Serour GI, Amin YM. Moderate ovarian hyperstimulation syndrome complicated by deep cerebrovascular thrombosis. Human Reproduction 1998;13(8):2088‐91. - PubMed
Al‐Inany 2005
-
- Al‐Inany H, Aboulghar MA, Mansour RT, Proctor M. Recombinant versus urinary gonadotrophins for triggering ovulation in assisted conception. Human Reproduction 2005;20(8):2061– 2073. - PubMed
Cha 1998
-
- Cha KY, Chian RC. Maturation in vitro of immature human oocytes for clinical use. Human Reprodtion Update 1998;4(2):103‐20. - PubMed
Emperaire 1991
-
- Emperaire JC, Ruffie A. Triggering ovulation with endogenous luteinizing hormone may prevent the ovarian hyperstimulation syndrome. Human Reproduction 1991;6(4):506‐10. - PubMed
Fatemi 2012
-
- Fatemi HM, Blockeel C, Devroey P. Ovarian stimulation: today and tomorrow. Current Pharmaceutical Biotechnology 2012;13(3):392‐7. - PubMed
GRADEpro [Computer program]
-
- McMaster University. GRADEpro. Version 2016. Available from www.gradepro.org: McMaster University, 2014.
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. The Cochrane Collaboration, Available from www.cochrane‐handbook.org.
Leao 2014
Nastri 2015
-
- Nastri CO, Teixeira DM, Moroni RM, Leitao VMS, Martins WP. Ovarian hyperstimulation syndrome: pathophysiology, staging, prediction and prevention. Ultrasound in Obstetrics and Gynecology 2015;45(4):377‐93. - PubMed
Pierce 1981
-
- Pierce JG, Parsons TF. Glycoprotein hormones: structure and function. Annual Review of Biochemistry 1981;50:465‐95. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Trinchard‐Lugan 2002
-
- Trinchard‐Lugan I, Khan A, Porchet HC, Munafo A. Pharmacokinetics and pharmacodynamics of recombinant human chorionic gonadotrophin in healthy male and female volunteers. Reproductive Biomedicine Online 2002;4(2):106‐15. - PubMed
Zegers‐Hochschild 1996
-
- Zegers‐Hochschild F, Fernandez E, Mackenna A, Fabres C, Altieri E, Lopez T. The empty follicle syndrome: a pharmaceutical industry syndrome. Human Reproduction 1996;10(9):2262‐5. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

