Luteal versus follicular phase surgical oophorectomy plus tamoxifen in premenopausal women with metastatic hormone receptor-positive breast cancer
- PMID: 27107325
- PMCID: PMC5052674
- DOI: 10.1016/j.ejca.2016.03.011
Luteal versus follicular phase surgical oophorectomy plus tamoxifen in premenopausal women with metastatic hormone receptor-positive breast cancer
Abstract
Purpose: In premenopausal women with metastatic hormone receptor-positive breast cancer, hormonal therapy is the first-line therapy. Gonadotropin-releasing hormone analogue + tamoxifen therapies have been found to be more effective. The pattern of recurrence risk over time after primary surgery suggests that peri-operative factors impact recurrence. Secondary analyses of an adjuvant trial suggested that the luteal phase timing of surgical oophorectomy in the menstrual cycle simultaneous with primary breast surgery favourably influenced long-term outcomes.
Methods: Two hundred forty-nine premenopausal women with incurable or metastatic hormone receptor-positive breast cancer entered a trial in which they were randomised to historical mid-luteal or mid-follicular phase surgical oophorectomy followed by oral tamoxifen treatment. Kaplan-Meier methods, the log-rank test, and multivariable Cox regression models were used to assess overall and progression-free survival (PFS) in the two randomised groups and by hormone-confirmed menstrual cycle phase.
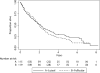
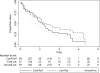
Results: Overall survival (OS) and PFS were not demonstrated to be different in the two randomised groups. In a secondary analysis, OS appeared worse in luteal phase surgery patients with progesterone levels <2 ng/ml (anovulatory patients; adjusted hazard ratio 1.46, 95% confidence interval [CI]: 0.89-2.41, p = 0.14) compared with those in luteal phase with progesterone level of 2 ng/ml or higher. Median OS was 2 years (95% CI: 1.7-2.3) and OS at 4 years was 26%.
Conclusions: The history-based timing of surgical oophorectomy in the menstrual cycle did not influence outcomes in this trial of metastatic patients. ClinicalTrials.gov number NCT00293540.
Keywords: Acute effects; Anovulatory; Metastatic; Oophorectomy.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
statement The authors indicated no potential conflicts of interest.
Figures

References
-
- Buzdar AU, Hortobagyi G. Update on endocrine therapy for breast cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 1998;4:527–34. - PubMed
-
- Fossati R, Confalonieri C, Torri V, et al. Cytotoxic and hormonal treatment for metastatic breast cancer: a systematic review of published randomized trials involving 31,510 women. J Clin Oncol. 1998;16:3439–60. - PubMed
-
- Ingle JN, Krook JE, Green SJ, et al. Randomized trial of bilateral oophorectomy versus tamoxifen in premenopausal women with metastatic breast cancer. J Clin Oncol. 1986;4:178–85. - PubMed
-
- Buchanan RB, Blamey RW, Durrant KR, et al. A randomized comparison of tamoxifen with surgical oophorectomy in premenopausal patients with advanced breast cancer. J Clin Oncol. 1986;4:1326–30. - PubMed
-
- Crump M, Sawka CA, DeBoer G, et al. An individual patient-based meta-analysis of tamoxifen versus ovarian ablation as first line endocrine therapy for premenopausal women with metastatic breast cancer. Breast Cancer Res Treat. 1997;44:201–10. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

