Low bacterial diet versus control diet to prevent infection in cancer patients treated with chemotherapy causing episodes of neutropenia
- PMID: 27107610
- PMCID: PMC6466670
- DOI: 10.1002/14651858.CD006247.pub3
Low bacterial diet versus control diet to prevent infection in cancer patients treated with chemotherapy causing episodes of neutropenia
Abstract
Background: Neutropenia is a potentially serious side effect of chemotherapy and a major risk factor for infection, which can be life-threatening. It has been hypothesised that a low bacterial diet (LBD) can prevent infection and (infection-related) mortality in cancer patients receiving chemotherapy that causes episodes of neutropenia, but much remains unclear. This review is an update of a previously published Cochrane review.
Objectives: The primary objective of this review was to determine the efficacy of an LBD versus a control diet in preventing infection and in decreasing (infection-related) mortality in adult and paediatric cancer patients receiving chemotherapy that causes episodes of neutropenia. Secondary objectives were to assess time to first febrile episode, need for empirical antibiotic therapy, diet acceptability and quality of life.
Search methods: We searched the following electronic databases: the Cochrane Central Register of Controlled Trials (CENTRAL) (2015, Issue 4), the Database of Abstracts of Reviews of Effects (DARE) (2015, Issue 4), PubMed (from 1946 to 4 May 2015), EMBASE (from 1980 to 4 May 2015) and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (from 1981 to 4 May 2015).In addition, we searched the reference lists of relevant articles and conference proceedings of American Society of Hematology (ASH; from 2000 to 2015), European Bone Marrow Transplantation (EBMT; from 2000 to 2015), Oncology Nurses Society (ONS; from 2000 to 2015), International Society for Paediatric Oncology (SIOP; from 2000 to 2014), Multinational Association of Supportive Care in Cancer (MASCC; from 2000 to 2015), American Society of Clinical Oncology (ASCO; from 2000 to 2015), Interscience Conference of Antimicrobial Agents and Chemotherapy (ICAAC; from 2000 to 2015), European Society for Clinical Nutrition and Metabolism (ESPEN; from 2000 to 2015), American Society for Parenteral and Enteral Nutrition (ASPEN; from 2000 to 2015) and European Hematology Association (EHA; from 2000 to 2015). In May 2015, we scanned the National Institutes of Health Register via clinicaltrials.gov and the International Standard Randomised Controlled Trial Number (ISRCTN) Register (www.controlled-trials.com).
Selection criteria: Randomised controlled trials (RCTs) comparing use of an LBD versus a control diet with regard to infection rate, (infection-related) mortality, time to first febrile episode, need for empirical antibiotic therapy, diet acceptability and quality of life in adult and paediatric cancer patients receiving chemotherapy causing episodes of neutropenia.
Data collection and analysis: Two review authors independently performed study selection, 'Risk of bias' assessment and data extraction. We performed analyses according to the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions.
Main results: In the original version of this review, we identified three RCTs that assessed different intervention and control diets in 192 participants (97 randomised to intervention diet; 95 to control diet) with different types of malignancies. For the update, we identified no eligible new studies. Co-interventions (e.g. protective environment, antimicrobial prophylaxis, central venous catheter care, oral care, hygiene practices, colony-stimulating factors) and outcome definitions also differed between studies. In all included studies, it was standard policy to give empirical antibiotics (and sometimes also antimycotics) to (some of) the participants diagnosed with an infection. Two studies included adults and one study included children. In all studies, only a scant description of treatment regimens was provided. All studies had methodological limitations. Pooling of results of included studies was not possible. In two individual studies, no statistically significant differences in infection rate were identified between intervention and control diets; another study showed no significant differences between treatment groups in the number of chemotherapy cycles with an infection. None of the studies mentioned infection-related mortality, but in one study, no significant difference in overall survival was observed between treatment groups. Time from onset of neutropenia to fever, duration of empirical antibiotics and antimycotics, diet acceptability (i.e. following the diet easily and following the diet throughout all chemotherapy cycles) and quality of life were all evaluated by only one study; for all outcomes, no statistically significant differences between treatment arms were identified.
Authors' conclusions: At the moment, no evidence from individual RCTs in children and adults with different malignancies underscores use of an LBD for prevention of infection and related outcomes. All studies differed with regard to co-interventions, outcome definitions and intervention and control diets. As pooling of results was not possible, and as all studies had serious methodological limitations, we could reach no definitive conclusions. It should be noted that 'no evidence of effect', as identified in this review, is not the same as 'evidence of no effect'. On the basis of currently available evidence, we are not able to provide recommendations for clinical practice. Additional high-quality research is needed.
Conflict of interest statement
None known.
Figures
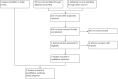







Update of
-
Low bacterial diet versus control diet to prevent infection in cancer patients treated with chemotherapy causing episodes of neutropenia.Cochrane Database Syst Rev. 2012 Sep 12;(9):CD006247. doi: 10.1002/14651858.CD006247.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2016 Apr 24;4:CD006247. doi: 10.1002/14651858.CD006247.pub3. PMID: 22972091 Updated.
References
References to studies included in this review
Gardner 2008 {published data only (unpublished sought but not used)}
Moody 2006 {published data only (unpublished sought but not used)}
-
- Moody K, Finlay J, Mancuso C, Charlson M. Feasibility and safety of a pilot randomized trial of infection rate: neutropenic diet versus standard food safety guidelines. Journal of Pediatric Hematology and Oncology 2006;28(3):126‐33. - PubMed
Van Tiel 2007 {published data only (unpublished sought but not used)}
-
- Tiel FH, Harbers MM, Terporten PHW, Boxtel RTC, Kessels AG, Voss GBWE, et al. Normal hospital and low‐bacterial diet in patients with cytopenia after intensive chemotherapy for hematological malignancy: a study of safety. Annals of Oncology 2007;18:1080‐4. - PubMed
References to studies excluded from this review
Brown 2014 {published data only}
-
- Brown LE, Chen H, Frangoul H. Significant inconsistency among pediatric oncologists in the use of the neutropenic diet. Pediatric Blood and Cancer 2014;61(10):1806‐10. - PubMed
Carr 2014 {published data only}
-
- Carr SE, Halliday V. Investigating the use of the neutropenic diet: a survey of UK dietitians. Journal of Human Nutrition and Dietetics 2014;28(5):510‐5. - PubMed
DeMille 2006 {published data only}
-
- DeMille D, Deming P, Lupinacci P, Jacobs LA. The effect of the neutropenic diet in the outpatient setting: a pilot study. Oncology Nursing Forum 2006;33(2):337‐43. - PubMed
Fopp 1975 {published data only}
-
- Fopp M, Gasser A, Eigenmann B, Jungi WF, Meuret G, Senn HJ. Prevention of infection in patients with agranulocytosis through isolation and whole‐body decontamination [Infektprophylaxe bei Patienten mit Agranulozytose durch Isolation und Ganzkörperdekontamination]. Schweizerische Medizinische Wochenschrift 1975;105(35):1123‐5. - PubMed
Foster 2014 {published data only}
-
- Foster M. Reevaluating the neutropenic diet: time to change. Clinical Journal of Oncology Nursing 2014;18(2):239‐41. - PubMed
Fox 2012 {published data only}
-
- Fox N, Freifeld AG. The neutropenic diet reviewed: moving toward a safe food handling approach. Oncology 2012;26(6):572‐5, 580, 582. - PubMed
Lund 2014 {published data only}
-
- Lund BM. Microbiological food safety and low‐bacterial diet to protect vulnerable people. Food Borne Pathogens and Disease 2014;11(6):413‐24. - PubMed
Trifilio 2012 {published data only}
-
- Trifilio S, Helenowski I, Giel M, Gobel B, Pi J, Greenberg D, et al. Questioning the role of neutropenic diet following hematopoietic stem cell transplantation. Biological Blood Marrow Transplantion 2012;18(9):1385‐90. - PubMed
Veber 2010 {published data only (unpublished sought but not used)}
-
- Veber Y, Kokunova E, Smirnova M, Ivanova O, Kucher M, Afanasyev B. Nutrition support in allogeneic stem cell transplantation. Comparison of standard and modified regimens, single‐centre experience. Bone Marrow Transplantation. 36th Annual Meeting of the European Group for Blood and Marrow Transplantation, EBMT; 2010 March 21‐24; Vienna, Austria. 2010; Vol. 45:S350.
Wilson 2002 {published data only}
-
- Wilson BJ. Dietary recommendations for neutropenic patients. Seminars in Oncology Nursing 2002;18(1):44‐9. - PubMed
Ziegler 1992 {published data only}
-
- Ziegler TR, Young LS, Benfell K, Scheltinga M, Hortos K, Bye R, et al. Clinical and metabolic efficacy of glutamine‐supplemented parenteral nutrition after bone marrow transplantation. A randomized, double‐blind, controlled study. Annals of Internal Medicine 1992;116(10):821‐8. - PubMed
References to studies awaiting assessment
Van 't Veer 1987 {published and unpublished data}
-
- Van't Veer‐Korthof ET, Dooren LJ, Guiot HFL, Palmer JG, Vossen JMJJ. Prevention of infections in granulocytopenic patients by a cooked‐food diet in combination with selective GI decontamination and partial protective isolation. Blood; 29th Annual Meeting of the American Society of Hematology; 1987 Dec 4‐8; Washington (DC). 1987; Vol. 70:239a.
References to ongoing studies
NCT00726934 {published data only}
-
- NCT00726934. The effectiveness of the neutropenic diet in pediatric oncology patients. http://clinicaltrials.gov/ct2/show/NCT00726934 (accessed 4 May 2015).
NCT00947648 {published data only}
-
- NCT00947648. Are neutropenic diets beneficial to improve outcome?. http://clinicaltrials.gov/ct2/show/NCT00947648 (accessed 4 May 2015).
Additional references
Aker 1983
-
- Aker SN, Cheney CL. The use of sterile and low microbial diets in ultra isolation environments. Journal of Parenteral and Enteral Nutrition 1983;7(4):390‐7. - PubMed
Barber 2001
-
- Barber FD. Management of fever in neutropenic patients with cancer. Nursing Clinics of North America 2001;36:631‐44. - PubMed
Candell 1991
-
- Candell KA, Whedon MB. Hematopoietic complications. In: MB Whedon editor(s). In: Bone Marrow Transplantation Principles, Practice, and Nursing Insights. Burlington, MA: Jones and Bartlett Publishers, 1991:136.
Dykewicz 2001
-
- Dykewicz CA. Summary of the guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. Clinical Infectious Diseases 2001;33(2):139‐44. - PubMed
Food Safety 2005
-
- Gateway to Government Food Safety Information. Advice for Consumers 2005. http://www.foodsafety.gov (accessed 4 May 2012).
Freifeld 2011
-
- Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clinical Infectious Diseases 2011;52(4):e56‐93. - PubMed
French 2001
-
- French MR, Levy‐Milne R, Zibrik D. A survey of the use of low microbial diets in pediatric bone marrow transplant programs. Journal of American Dietetic Association 2001;101(10):1194‐8. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org, 2011.
Hughes 2002
-
- Hughes WT, Armstrong D, Bodey GP, Bow EJ, Brown AE, Calandra T, et al. Guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clinical Infectious Diseases 2002;34(6):730‐51. - PubMed
Klastersky 2000
-
- Klastersky J, Paesmans M, Rubenstein EB, Boyer M, Elting L, Feld R, et al. Multinational Association for Supportive Care in Cancer risk index: a multinational scoring system for identifying low‐risk febrile neutropenic cancer patients. Journal of Clinical Oncology 2000;18(16):3038‐51. - PubMed
Lachin 2000
-
- Lachin JM. Statistical considerations in the intent‐to‐treat principle. Controlled Clinical Trials 2000;21:167‐89. - PubMed
Larson 2004
-
- Larson E, Nirenberg A. Evidence‐based nursing practice to prevent infection in hospitalized neutropenic patients with cancer. Oncology Nursing Forum 2004;31(4):717‐25. - PubMed
MacVittie 1997
-
- MacVittie TJ. Therapy of radiation injury. Stem Cells 1997;15(Suppl 2):263‐8. - PubMed
Module CCG 2010
-
- Kremer LCM, Dalen EC, Moher D, Caron HN. Cochrane Childhood Cancer Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)) 2010, issue 1:Art. No.: CHILDCA.
Module GCG 2010
-
- Williams C, Quinn G, Jess C, Bishop T, Hayes J. Cochrane Gynaecological Cancer Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)) 2010, issue 4:Art. No.: GYNAECA.
Moody 2002
-
- Moody K, Charlson ME, Finlay J. The neutropenic diet: what's the evidence?. Journal Pediatric Hematological Oncology 2002;24(9):717‐21. - PubMed
Pizzo 1982
-
- Pizzo PA, Purvis DS, Waters C. Microbiological evaluation of food items for patients undergoing gastrointestinal decontamination and protected isolation. Journal of the American Dietetic Association 1982;81(3):272‐9. - PubMed
Pizzo 1999
-
- Pizzo PA. Fever in immunocompromised patients. New England Journal of Medicine 1999;341(12):893‐900. - PubMed
Preisler 1970
-
- Preisler HD, Goldstein IM, Henderson ES. Gastrointestinal "sterilization" in the treatment of patients with acute leukemia. Cancer 1970;26(5):1076‐81. - PubMed
Reimer 1966
-
- Reimer AO, Tillotson JL. Food service procedures for reverse isolation. Journal of the American Dietetic Association 1966;48(5):381‐4. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3.5. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roguin 1996
-
- Roguin A, Kasis I, Ben‐Arush MW, Sharon R, Berant M. Fever and neutropenia in children with malignant disease. Pediatric Hematology and Oncology 1996;13(6):503‐10. - PubMed
Smith 2000
-
- Smith LH, Besser SG. Dietary restrictions for patients with neutropenia: a survey of institutional practices. Oncology Nursing Forum 2000;27(3):515‐20. - PubMed
Talcott 1992
-
- Talcott JA, Siegel RD, Finberg R, Goldman L. Risk assessment in cancer patients with fever and neutropenia: a prospective, two‐center validation of a prediction rule. Journal of Clinical Oncology 1992;10(2):316‐22. - PubMed
Varni 2002
-
- Varni JW, Burwinkle TM, Katz ER, Meeske K, Dickinson P. The PedsQL in pediatric cancer: reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales, Multidimensional Fatigue Scale, and Cancer Module. Cancer 2002;94(7):2090‐106. - PubMed
References to other published versions of this review
Mank 2006
-
- Mank A, Davies M, Langeveld N, Wetering MD, Lelie H. Low bacterial diet to prevent infection in neutropenic patients. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD006247] - DOI
Mank 2012
-
- Dalen EC, Mank A, Leclercq E, Mulder RL, Davies M, Kersten MJ, Wetering MD. Low bacterial diet versus control diet to prevent infection in cancer patients treated with chemotherapy causing episodes of neutropenia. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD006247.pub2] - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

