A Restrictive Cardiomyopathy Mutation in an Invariant Proline at the Myosin Head/Rod Junction Enhances Head Flexibility and Function, Yielding Muscle Defects in Drosophila
- PMID: 27107639
- PMCID: PMC4884507
- DOI: 10.1016/j.jmb.2016.04.021
A Restrictive Cardiomyopathy Mutation in an Invariant Proline at the Myosin Head/Rod Junction Enhances Head Flexibility and Function, Yielding Muscle Defects in Drosophila
Abstract
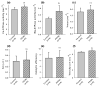
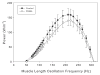
An "invariant proline" separates the myosin S1 head from its S2 tail and is proposed to be critical for orienting S1 during its interaction with actin, a process that leads to muscle contraction. Mutation of the invariant proline to leucine (P838L) caused dominant restrictive cardiomyopathy in a pediatric patient (Karam et al., Congenit. Heart Dis. 3:138-43, 2008). Here, we use Drosophila melanogaster to model this mutation and dissect its effects on the biochemical and biophysical properties of myosin, as well as on the structure and physiology of skeletal and cardiac muscles. P838L mutant myosin isolated from indirect flight muscles of transgenic Drosophila showed elevated ATPase and actin sliding velocity in vitro. Furthermore, the mutant heads exhibited increased rotational flexibility, and there was an increase in the average angle between the two heads. Indirect flight muscle myofibril assembly was minimally affected in mutant homozygotes, and isolated fibers displayed normal mechanical properties. However, myofibrils degraded during aging, correlating with reduced flight abilities. In contrast, hearts from homozygotes and heterozygotes showed normal morphology, myofibrillar arrays, and contractile parameters. When P838L was placed in trans to Mhc(5), an allele known to cause cardiac restriction in flies, it did not yield the constricted phenotype. Overall, our studies suggest that increased rotational flexibility of myosin S1 enhances myosin ATPase and actin sliding. Moreover, instability of P838L myofibrils leads to decreased function during aging of Drosophila skeletal muscle, but not cardiac muscle, despite the strong evolutionary conservation of the P838 residue.
Keywords: Drosophila melanogaster; contraction; electron microscopy; motility; myofibril.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures




References
-
- Hoedemaekers YM, Caliskan K, Majoor-Krakauer D, van de Laar I, Michels M, Witsenburg M, et al. Cardiac beta-myosin heavy chain defects in two families with non-compaction cardiomyopathy: linking non-compaction to hypertrophic, restrictive, and dilated cardiomyopathies. Eur Heart J. 2007;28:2732–2737. - PubMed
-
- Rai TS, Ahmad S, Ahluwalia TS, Ahuja M, Bahl A, Saikia UN, et al. Genetic and clinical profile of Indian patients of idiopathic restrictive cardiomyopathy with and without hypertrophy. Mol Cell Biochem. 2009;331:187–192. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

