Dual oxidase 1: A predictive tool for the prognosis of hepatocellular carcinoma patients
- PMID: 27108801
- PMCID: PMC4869938
- DOI: 10.3892/or.2016.4745
Dual oxidase 1: A predictive tool for the prognosis of hepatocellular carcinoma patients
Abstract
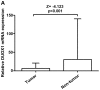
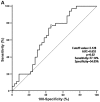
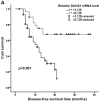
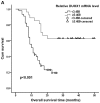
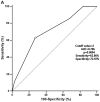
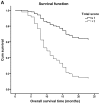
Dual oxidase 1 (DUOX1), which is the main source of reactive oxygen species (ROS) production in the airway, can be silenced in human lung cancer and hepatocellular carcinomas. However, the prognostic value of DUOX1 expression in hepatocellular carcinoma patients is still unclear. We investigated the prognostic value of DUOX1 expression in liver cancer patients. DUOX1 mRNA expression was determined in tumor tissues and non-tumor tissues by real‑time PCR. For evaluation of the prognostic value of DUOX1 expression, Kaplan-Meier method and Cox's proportional hazards model (univariate analysis and multivariate analysis) were employed. A simple risk score was devised by using significant variables obtained from the Cox's regression analysis to further predict the HCC patient prognosis. We observed a reduced DUOX1 mRNA level in the cancer tissues in comparison to the non‑cancer tissues. More importantly, Kaplan-Meier analysis showed that patients with high DUOX1 expression had longer disease-free survival and overall survival compared with those with low expression of DUOX1. Cox's regression analysis indicated that DUOX1 expression, age, and intrahepatic metastasis may be significant prognostic factors for disease-free survival and overall survival. Finally, we found that patients with total scores of >2 and >1 were more likely to relapse and succumb to the disease than patients whose total scores were ≤2 and ≤1. In conclusion, DUOX1 expression in liver tumors is a potential prognostic tool for patients. The risk scoring system is useful for predicting the survival of liver cancer patients after tumor resection.
Figures



















References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

