Hypoxia-induced oxidative stress promotes MUC4 degradation via autophagy to enhance pancreatic cancer cells survival
- PMID: 27109098
- PMCID: PMC5079846
- DOI: 10.1038/onc.2016.119
Hypoxia-induced oxidative stress promotes MUC4 degradation via autophagy to enhance pancreatic cancer cells survival
Abstract
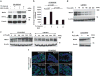
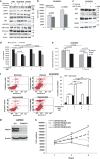
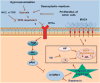
Pancreatic cancer (PC) and associated pre-neoplastic lesions have been reported to be hypoxic, primarily due to hypovascular nature of PC. Though the presence of hypoxia under cancerous condition has been associated with the overexpression of oncogenic proteins (MUC1), multiple emerging reports have also indicated the growth inhibitory effects of hypoxia. In spite of being recognized as the top-most differentially expressed and established oncogenic protein in PC, MUC4 regulation in terms of micro-environmental stress has not been determined. Herein, for the first time, we are reporting that MUC4 protein stability is drastically affected in PC, under hypoxic condition in a hypoxia inducible factor 1α (HIF-1α)-independent manner. Mechanistically, we have demonstrated that hypoxia-mediated induction of reactive oxygen species (ROS) promotes autophagy by inhibiting pAkt/mTORC1 pathway, one of the central regulators of autophagy. Immunohistofluorescence analyses revealed significant negative correlation (P-value=0.017) between 8-hydroxy guanosine (8-OHG) and MUC4 in primary pancreatic tumors (n=25). Moreover, we found pronounced colocalization between MUC4 and LAMP1/LC3 (microtubule-associated protein 1A/1B-light chain 3) in PC tissues and also observed their negative relationship in their expression pattern, suggesting that areas with high autophagy rate had less MUC4 expression. We also found that hypoxia and ROS have negative impact on overall cell growth and viability, which was partially, though significantly (P<0.05), rescued in the presence of MUC4. Altogether, hypoxia-mediated oxidative stress induces autophagy in PC, leading to the MUC4 degradation to enhance survival, possibly by offering required metabolites to stressed cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. - PubMed
-
- Sullivan R, Pare GC, Frederiksen LJ, Semenza GL, Graham CH. Hypoxia-induced resistance to anticancer drugs is associated with decreased senescence and requires hypoxia-inducible factor-1 activity. Mol Cancer Ther. 2008;7:1961–1973. - PubMed
-
- Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, et al. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379:88–91. - PubMed
-
- Andrianifahanana M, Moniaux N, Schmied BM, Ringel J, Friess H, Hollingsworth MA, et al. Mucin (MUC) gene expression in human pancreatic adenocarcinoma and chronic pancreatitis: a potential role of MUC4 as a tumor marker of diagnostic significance. Clin Cancer Res. 2001;7:4033–4040. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

