Crosstalk between bone marrow-derived myofibroblasts and gastric cancer cells regulates cancer stemness and promotes tumorigenesis
- PMID: 27109105
- PMCID: PMC5063653
- DOI: 10.1038/onc.2016.76
Crosstalk between bone marrow-derived myofibroblasts and gastric cancer cells regulates cancer stemness and promotes tumorigenesis
Abstract
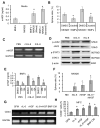
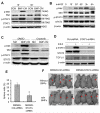
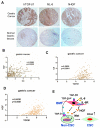
Bone marrow-derived cells have important roles in cancer development and progression. Our previous studies demonstrated that murine bone marrow-derived myofibroblasts (BMFs) enhanced tumor growth. In this study, we investigated the mechanisms of BMF actions. We found that co-injection of BMFs with gastric cancer cells markedly promoted tumorigenesis. Co-cultured BMFs or BMF-conditioned medium (BMF-CM) induced the formation of spheres, which expressed stem cell signatures and exhibited features of self-renewal, epithelial-to-mesenchymal transition and tumor initiation. Furthermore, CD44+ fractions in spheres were able to initiate tumorigenesis and re-establish tumors in serially passaged xenografts. In co-culture systems, BMFs secreted high levels of murine interleukin-6 (IL-6) and hepatocyte growth factor (HGF), whereas cancer cells produced high level of transformation growth factor-β1 (TGF-β1). BMF-CM and IL-6 activated BMFs to produce mHGF, which activated signal transducer and activator of transcription 3 (STAT3) and upregulated TGF-β1 in human cancer cells. In return, cancer cell-CM stimulated BMFs to produce IL-6, which was inhibited by anti-TGF-β1 neutralizing antibody. Blockade of HGF/Met, Janus kinase 2 (JAK2)/STAT3 and TGF-β1 signaling by specific inhibitors inhibited BMF-induced sphere formation. STAT3 knockdown in cancer cells also inhibited BMF-induced sphere formation and tumorigenesis. Moreover, TGF-β1 overexpression in cancer cells was co-related with IL-6 and HGF overexpression in stromal cells in human gastric cancer tissues. Our results show that BMF-derived IL-6/HGF and cancer cell-derived TGF-β1 mediate the interactions between BMFs and gastric cancer cells, which regulate cancer stemness and promote tumorigenesis. Targeting inhibition of the interactions between BMFs and cancer cells may be a new strategy for cancer therapy.
Figures




Similar articles
-
Bone marrow-derived myofibroblasts promote colon tumorigenesis through the IL-6/JAK2/STAT3 pathway.Cancer Lett. 2014 Feb 1;343(1):80-9. doi: 10.1016/j.canlet.2013.09.017. Epub 2013 Oct 18. Cancer Lett. 2014. PMID: 24145153
-
Bone Marrow-Derived Myofibroblasts Promote Gastric Cancer Metastasis by Activating TGF-β1 and IL-6/STAT3 Signalling Loop.Onco Targets Ther. 2020 Oct 16;13:10567-10580. doi: 10.2147/OTT.S266506. eCollection 2020. Onco Targets Ther. 2020. PMID: 33116635 Free PMC article.
-
Targeted blockade of TGF-β and IL-6/JAK2/STAT3 pathways inhibits lung cancer growth promoted by bone marrow-derived myofibroblasts.Sci Rep. 2017 Aug 17;7(1):8660. doi: 10.1038/s41598-017-09020-8. Sci Rep. 2017. PMID: 28819126 Free PMC article.
-
Reciprocal functions of hepatocyte growth factor and transforming growth factor-beta1 in the progression of renal diseases: a role for CD44?Kidney Int Suppl. 2003 Oct;(86):S15-20. doi: 10.1046/j.1523-1755.64.s86.4.x. Kidney Int Suppl. 2003. PMID: 12969122 Review.
-
Angiogenic factors: role in esophageal cancer, a brief review.Esophagus. 2018 Apr;15(2):53-58. doi: 10.1007/s10388-017-0597-1. Epub 2017 Dec 16. Esophagus. 2018. PMID: 29892930 Review.
Cited by
-
Dissection of gastric cancer heterogeneity for precision oncology.Cancer Sci. 2019 Nov;110(11):3405-3414. doi: 10.1111/cas.14191. Epub 2019 Sep 25. Cancer Sci. 2019. PMID: 31495054 Free PMC article. Review.
-
Immunological Aspects of the Tumor Microenvironment and Epithelial-Mesenchymal Transition in Gastric Carcinogenesis.Int J Mol Sci. 2020 Apr 6;21(7):2544. doi: 10.3390/ijms21072544. Int J Mol Sci. 2020. PMID: 32268527 Free PMC article. Review.
-
Stromal Myofibroblasts Are Associated with Poor Prognosis in Solid Cancers: A Meta-Analysis of Published Studies.PLoS One. 2016 Jul 26;11(7):e0159947. doi: 10.1371/journal.pone.0159947. eCollection 2016. PLoS One. 2016. PMID: 27459365 Free PMC article.
-
Stroma-derived IL-6, G-CSF and Activin-A mediated dedifferentiation of lung carcinoma cells into cancer stem cells.Sci Rep. 2018 Aug 1;8(1):11573. doi: 10.1038/s41598-018-29947-w. Sci Rep. 2018. PMID: 30069023 Free PMC article.
-
8-bromo-7-methoxychrysin suppress stemness of SMMC-7721 cells induced by co-culture of liver cancer stem-like cells with hepatic stellate cells.BMC Cancer. 2019 Mar 12;19(1):224. doi: 10.1186/s12885-019-5419-5. BMC Cancer. 2019. Retraction in: BMC Cancer. 2023 Dec 7;23(1):1203. doi: 10.1186/s12885-023-11698-1. PMID: 30866863 Free PMC article. Retracted.
References
-
- Double blind controlled phase III multicenter clinical trial with interferon gamma in rheumatoid arthritis. German Lymphokine Study Group. Rheumatol Int. 1992;12:175–185. - PubMed
-
- Beppu H, Mwizerwa ON, Beppu Y, Dattwyler MP, Lauwers GY, Bloch KD, et al. Stromal inactivation of BMPRII leads to colorectal epithelial overgrowth and polyp formation. Oncogene. 2008;27:1063–1070. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

