Biofluorescence in Catsharks (Scyliorhinidae): Fundamental Description and Relevance for Elasmobranch Visual Ecology
- PMID: 27109385
- PMCID: PMC4843165
- DOI: 10.1038/srep24751
Biofluorescence in Catsharks (Scyliorhinidae): Fundamental Description and Relevance for Elasmobranch Visual Ecology
Abstract
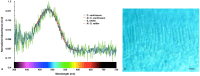
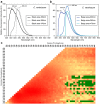
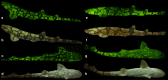
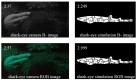
Biofluorescence has recently been found to be widespread in marine fishes, including sharks. Catsharks, such as the Swell Shark (Cephaloscyllium ventriosum) from the eastern Pacific and the Chain Catshark (Scyliorhinus retifer) from the western Atlantic, are known to exhibit bright green fluorescence. We examined the spectral sensitivity and visual characteristics of these reclusive sharks, while also considering the fluorescent properties of their skin. Spectral absorbance of the photoreceptor cells in these sharks revealed the presence of a single visual pigment in each species. Cephaloscyllium ventriosum exhibited a maximum absorbance of 484 ± 3 nm and an absorbance range at half maximum (λ1/2max) of 440-540 nm, whereas for S. retifer maximum absorbance was 488 ± 3 nm with the same absorbance range. Using the photoreceptor properties derived here, a "shark eye" camera was designed and developed that yielded contrast information on areas where fluorescence is anatomically distributed on the shark, as seen from other sharks' eyes of these two species. Phylogenetic investigations indicate that biofluorescence has evolved at least three times in cartilaginous fishes. The repeated evolution of biofluorescence in elasmobranchs, coupled with a visual adaptation to detect it; and evidence that biofluorescence creates greater luminosity contrast with the surrounding background, highlights the potential importance of biofluorescence in elasmobranch behavior and biology.
Figures










References
-
- Gruber D. F., Kao H.-T., Janoschka S., Tsai J. & Pieribone V. a. Patterns of fluorescent protein expression in Scleractinian corals. Biol. Bull. 215, 143–154 (2008). - PubMed
-
- Gruber D. F. & Sparks J. S. First Observation of Fluorescence in Marine Turtles. Am. Museum Novit. 3845, 1–8 (2015).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

