Dual Catalysis Strategies in Photochemical Synthesis
- PMID: 27109441
- PMCID: PMC5083252
- DOI: 10.1021/acs.chemrev.6b00018
Dual Catalysis Strategies in Photochemical Synthesis
Abstract
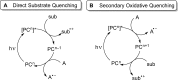
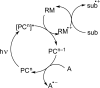
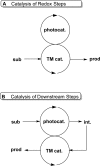
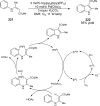
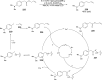
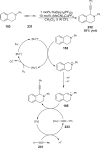
The interaction between an electronically excited photocatalyst and an organic molecule can result in the genertion of a diverse array of reactive intermediates that can be manipulated in a variety of ways to result in synthetically useful bond constructions. This Review summarizes dual-catalyst strategies that have been applied to synthetic photochemistry. Mechanistically distinct modes of photocatalysis are discussed, including photoinduced electron transfer, hydrogen atom transfer, and energy transfer. We focus upon the cooperative interactions of photocatalysts with redox mediators, Lewis and Brønsted acids, organocatalysts, enzymes, and transition metal complexes.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



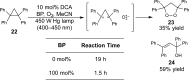





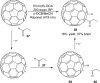
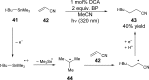
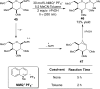

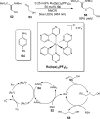

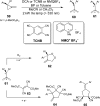
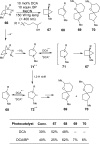
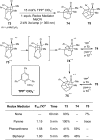






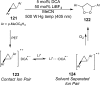

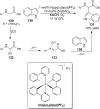
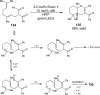

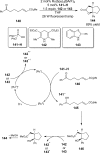
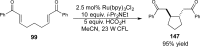

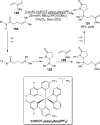
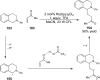


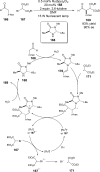
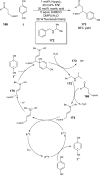
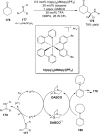



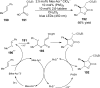

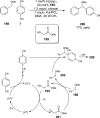
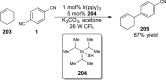

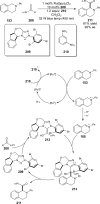
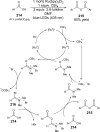
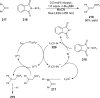


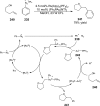


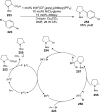
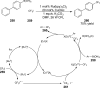

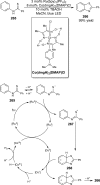

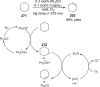
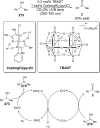



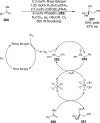

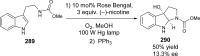

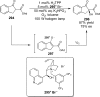
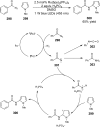

References
-
- Albini A.; Fagnoni M.. Photochemically Generated Intermediates in Synthesis; Wiley & Sons: Hoboken, 2013.
-
- Kavarnos G. J.; Turro N. J. Photosensitization by Reversible Electron Transfer: Theories, Experimental Evidence, and Examples. Chem. Rev. 1986, 86, 401–449. 10.1021/cr00072a005. - DOI
-
- Scaiano J. C. Intermolecular Photoreductions of Ketones. J. Photochem. 1973, 2, 81–118. 10.1016/0047-2670(73)80010-3. - DOI
-
- Turro N. J. Energy Transfer Processes. Pure Appl. Chem. 1977, 49, 405–429. 10.1016/B978-0-08-021205-0.50005-1. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

