A Class of Rigid Linker-bearing Glucosides for Membrane Protein Structural Study
- PMID: 27110345
- PMCID: PMC4836865
- DOI: 10.1039/C5SC02900G
A Class of Rigid Linker-bearing Glucosides for Membrane Protein Structural Study
Abstract
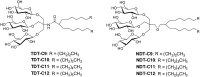
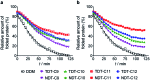
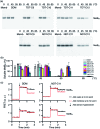
Membrane proteins are amphipathic bio-macromolecules incompatible with the polar environments of aqueous media. Conventional detergents encapsulate the hydrophobic surfaces of membrane proteins allowing them to exist in aqueous solution. Membrane proteins stabilized by detergent micelles are used for structural and functional analysis. Despite the availability of a large number of detergents, only a few agents are sufficiently effective at maintaining the integrity of membrane proteins to allow successful crystallization. In the present study, we describe a novel class of synthetic amphiphiles with a branched tail group and a triglucoside head group. These head and tail groups were connected via an amide or ether linkage by using a tris(hydroxylmethyl)aminomethane (TRIS) or neopentyl glycol (NPG) linker to produce TRIS-derived triglucosides (TDTs) and NPG-derived triglucosides (NDTs), respectively. Members of this class conferred enhanced stability on target membrane proteins compared to conventional detergents. Because of straightforward synthesis of the novel agents and their favourable effects on a range of membrane proteins, these agents should be of wide applicability to membrane protein science.
Figures

Similar articles
-
Tris(hydroxymethyl)aminomethane Linker-Bearing Triazine-Based Triglucosides for Solubilization and Stabilization of Membrane Proteins.Bioconjug Chem. 2023 Apr 19;34(4):739-747. doi: 10.1021/acs.bioconjchem.3c00042. Epub 2023 Mar 15. Bioconjug Chem. 2023. PMID: 36919927 Free PMC article.
-
Highly Branched Pentasaccharide-Bearing Amphiphiles for Membrane Protein Studies.J Am Chem Soc. 2016 Mar 23;138(11):3789-96. doi: 10.1021/jacs.5b13233. Epub 2016 Mar 11. J Am Chem Soc. 2016. PMID: 26966956 Free PMC article.
-
Unsymmetric Triazine-Based Triglucoside Detergents for Membrane Protein Stability.Chembiochem. 2025 Feb 3;26(5):e202400958. doi: 10.1002/cbic.202400958. Epub 2025 Jan 28. Chembiochem. 2025. PMID: 39779472 Free PMC article.
-
Overcoming bottlenecks in the membrane protein structural biology pipeline.Biochem Soc Trans. 2016 Jun 15;44(3):838-44. doi: 10.1042/BST20160049. Biochem Soc Trans. 2016. PMID: 27284049 Review.
-
Strategies for crystallizing membrane proteins.J Bioenerg Biomembr. 1996 Feb;28(1):13-27. J Bioenerg Biomembr. 1996. PMID: 8786233 Review.
Cited by
-
Development of 1,3-acetonedicarboxylate-derived glucoside amphiphiles (ACAs) for membrane protein study.Chem Sci. 2022 Apr 19;13(19):5750-5759. doi: 10.1039/d2sc00539e. eCollection 2022 May 18. Chem Sci. 2022. PMID: 35694361 Free PMC article.
-
Thermodynamic cooperativity of cosubstrate binding and cation selectivity of Salmonella typhimurium MelB.J Gen Physiol. 2017 Nov 6;149(11):1029-1039. doi: 10.1085/jgp.201711788. Epub 2017 Oct 20. J Gen Physiol. 2017. PMID: 29054867 Free PMC article.
-
Conformationally Preorganized Diastereomeric Norbornane-Based Maltosides for Membrane Protein Study: Implications of Detergent Kink for Micellar Properties.J Am Chem Soc. 2017 Mar 1;139(8):3072-3081. doi: 10.1021/jacs.6b11997. Epub 2017 Feb 20. J Am Chem Soc. 2017. PMID: 28218862 Free PMC article.
-
Glyco-Steroidal Amphiphiles (GSAs) for Membrane Protein Structural Study.Chembiochem. 2022 Apr 5;23(7):e202200027. doi: 10.1002/cbic.202200027. Epub 2022 Feb 21. Chembiochem. 2022. PMID: 35129249 Free PMC article.
-
Accessible Mannitol-Based Amphiphiles (MNAs) for Membrane Protein Solubilisation and Stabilisation.Chemistry. 2016 May 17;22(21):7068-73. doi: 10.1002/chem.201600533. Epub 2016 Apr 13. Chemistry. 2016. PMID: 27072057 Free PMC article.
References
-
- Rosenbaum D. M., Rasmussen S. G. F., Kobilka B. K. Nature. 2009;459:356–363. - PMC - PubMed
- McCusker E. C., Bagneris C., Naylor C. E., Cole A. R., D'Avanzo N., Nichols C. G., Wallace B. A. Nat. Commun. 2012;3:1102. - PMC - PubMed
- Ghosh E., Kumari P., Jiaman D., Shukla A. K. Nat. Rev. Mol. Cell Biol. 2015;16:69–81. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

