White-to-brite conversion in human adipocytes promotes metabolic reprogramming towards fatty acid anabolic and catabolic pathways
- PMID: 27110487
- PMCID: PMC4837301
- DOI: 10.1016/j.molmet.2016.03.002
White-to-brite conversion in human adipocytes promotes metabolic reprogramming towards fatty acid anabolic and catabolic pathways
Abstract
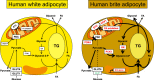
Objective: Fat depots with thermogenic activity have been identified in humans. In mice, the appearance of thermogenic adipocytes within white adipose depots (so-called brown-in-white i.e., brite or beige adipocytes) protects from obesity and insulin resistance. Brite adipocytes may originate from direct conversion of white adipocytes. The purpose of this work was to characterize the metabolism of human brite adipocytes.
Methods: Human multipotent adipose-derived stem cells were differentiated into white adipocytes and then treated with peroxisome proliferator-activated receptor (PPAR)γ or PPARα agonists between day 14 and day 18. Gene expression profiling was determined using DNA microarrays and RT-qPCR. Variations of mRNA levels were confirmed in differentiated human preadipocytes from primary cultures. Fatty acid and glucose metabolism was investigated using radiolabelled tracers, Western blot analyses and assessment of oxygen consumption. Pyruvate dehydrogenase kinase 4 (PDK4) knockdown was achieved using siRNA. In vivo, wild type and PPARα-null mice were treated with a β3-adrenergic receptor agonist (CL316,243) to induce appearance of brite adipocytes in white fat depot. Determination of mRNA and protein levels was performed on inguinal white adipose tissue.
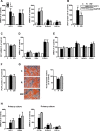
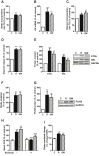
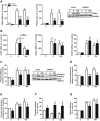
Results: PPAR agonists promote a conversion of white adipocytes into cells displaying a brite molecular pattern. This conversion is associated with transcriptional changes leading to major metabolic adaptations. Fatty acid anabolism i.e., fatty acid esterification into triglycerides, and catabolism i.e., lipolysis and fatty acid oxidation, are increased. Glucose utilization is redirected from oxidation towards glycerol-3-phophate production for triglyceride synthesis. This metabolic shift is dependent on the activation of PDK4 through inactivation of the pyruvate dehydrogenase complex. In vivo, PDK4 expression is markedly induced in wild-type mice in response to CL316,243, while this increase is blunted in PPARα-null mice displaying an impaired britening response.
Conclusions: Conversion of human white fat cells into brite adipocytes results in a major metabolic reprogramming inducing fatty acid anabolic and catabolic pathways. PDK4 redirects glucose from oxidation towards triglyceride synthesis and favors the use of fatty acids as energy source for uncoupling mitochondria.
Keywords: Brite/beige adipocyte; Fatty acid metabolism; Glycerol metabolism; Peroxisome proliferator-activated receptor; Pyruvate dehydrogenase kinase 4.
Figures


References
-
- Cannon B., Nedergaard J. Brown adipose tissue: function and physiological significance. Physiological Reviews. 2004;84:277–359. - PubMed
-
- Feldmann H.M., Golozoubova V., Cannon B., Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metabolism. 2009;9:203–209. - PubMed
-
- van Marken Lichtenbelt W.D., Vanhommerig J.W., Smulders N.M., Drossaerts J.M., Kemerink G.J., Bouvy N.D. Cold-activated brown adipose tissue in healthy men. The New England Journal of Medicine. 2009;360:1500–1508. - PubMed
-
- Virtanen K.A., Lidell M.E., Orava J., Heglind M., Westergren R., Niemi T. Functional brown adipose tissue in healthy adults. The New England Journal of Medicine. 2009;360:1518–1525. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

