Risk for Hospitalized Heart Failure Among New Users of Saxagliptin, Sitagliptin, and Other Antihyperglycemic Drugs: A Retrospective Cohort Study
- PMID: 27110660
- PMCID: PMC5178978
- DOI: 10.7326/M15-2568
Risk for Hospitalized Heart Failure Among New Users of Saxagliptin, Sitagliptin, and Other Antihyperglycemic Drugs: A Retrospective Cohort Study
Erratum in
-
Correction: Risk for Hospitalized Heart Failure Among New Users of Saxagliptin, Sitagliptin, and Other Antihyperglycemic Drugs.Ann Intern Med. 2016 Aug 16;165(4):304. doi: 10.7326/L16-0375. Ann Intern Med. 2016. PMID: 27538170 No abstract available.
Abstract
Background: Recent postmarketing trials produced conflicting results about the risk for hospitalized heart failure (hHF) associated with dipeptidyl peptidase-4 (DPP-4) inhibitors, creating uncertainty about the safety of these antihyperglycemic agents.
Objective: To examine the associations of hHF with saxagliptin and sitagliptin.
Design: Population-based, retrospective, new-user cohort study.
Setting: 18 health insurance and health system data partners in the U.S. Food and Drug Administration's Mini-Sentinel program.
Patients: Patients aged 18 years or older with type 2 diabetes who initiated therapy with saxagliptin, sitagliptin, pioglitazone, second-generation sulfonylureas, or long-acting insulin products from 2006 to 2013.
Measurements: Hospitalized HF, identified by International Classification of Diseases, Ninth Revision, Clinical Modification codes 402.x1, 404.x1, 404.x3, and 428.xx recorded as the principal discharge diagnosis.
Results: 78 553 saxagliptin users and 298 124 sitagliptin users contributed an average of 7 to 9 months of follow-up data to 1 or more pairwise comparisons. The risk for hHF was not higher with DPP-4 inhibitors than with the other study drugs. The hazard ratios from the disease risk score (DRS)-stratified analyses were 0.83 (95% CI, 0.70 to 0.99) for saxagliptin versus sitagliptin, 0.63 (CI, 0.47 to 0.85) for saxagliptin versus pioglitazone, 0.69 (CI, 0.54 to 0.87) for saxagliptin versus sulfonylureas, and 0.61 (CI, 0.50 to 0.73) for saxagliptin versus insulin. The DRS-stratified hazard ratios were 0.74 (CI, 0.64 to 0.85) for sitagliptin versus pioglitazone, 0.86 (CI, 0.77 to 0.95) for sitagliptin versus sulfonylureas, and 0.71 (CI, 0.64 to 0.78) for sitagliptin versus insulin. Results from the 1:1 propensity score-matched analyses were similar. Results were also similar in subgroups of patients with and without prior cardiovascular disease and in a subgroup defined by the 2 highest DRS deciles.
Limitation: Residual confounding and short follow-up.
Conclusion: In this large cohort study, a higher risk for hHF was not observed in users of saxagliptin or sitagliptin compared with other selected antihyperglycemic agents.
Primary funding source: U.S. Food and Drug Administration.
Conflict of interest statement
Dr. Toh reports grants from the U.S. Food and Drug Administration during the conduct of the study. Mr. Hamilton reports grants from the U.S. Food and Drug Administration during the conduct of the study. Dr. Lendle reports grants from the U.S. Food and Drug Administration during the conduct of the study. Dr. Iyer reports grants from the U.S. Food and Drug Administration during the conduct of the study. Mr. Rucker reports grants from the U.S. Food and Drug Administration during the conduct of the study. Ms. Pimentel reports grants from the U.S. Food and Drug Administration during the conduct of the study. Ms. Nathwani reports grants from the U.S. Food and Drug Administration during the conduct of the study. Dr. Griffin reports grants from the U.S. Food and Drug Administration and Harvard Pilgrim Health Care during the conduct of the study. Dr. Brown reports personal fees from Novartis Pharmaceuticals outside the submitted work. Mr. Fireman reports grants from the U.S. Food and Drug Administration during the conduct of the study. Authors not named here have disclosed no conflicts of interest. Disclosures can also be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M15-2568.
Figures


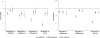
Comment in
-
Complementary Efforts Make for Efficient Research.Ann Intern Med. 2016 Jun 7;164(11):771-2. doi: 10.7326/M16-0869. Epub 2016 May 3. Ann Intern Med. 2016. PMID: 27110867 No abstract available.
References
-
- Zannad F, Cannon CP, Cushman WC, Bakris GL, Menon V, Perez AT, et al. EXAMINE Investigators Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067–76. doi: 10.1016/S0140-6736(14)62225-X. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
