A Multicenter, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of Treatment With Sirukumab (CNTO 136) in Patients With Active Lupus Nephritis
- PMID: 27110697
- PMCID: PMC5129491
- DOI: 10.1002/art.39722
A Multicenter, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of Treatment With Sirukumab (CNTO 136) in Patients With Active Lupus Nephritis
Abstract
Objective: To assess the efficacy and safety of sirukumab, an anti-interleukin-6 monoclonal antibody, for the treatment of patients with active lupus nephritis (LN).
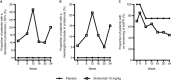
Methods: Patients with class III or class IV LN (as determined by renal biopsy within 14 months of randomization) who had persistent proteinuria (>0.5 gm/day) despite receiving immunosuppressive therapy and who were being treated with stable doses of a renin-angiotensin system blocker were randomized (5:1) to receive treatment with sirukumab at a dose of 10 mg/kg intravenously (n = 21) or placebo (n = 4) every 4 weeks through week 24. The primary end point was the percent reduction in proteinuria (measured as the protein-to-creatinine [P:C] ratio in a 12-hour urine collection) from baseline to week 24.
Results: Twenty-five patients were enrolled, of whom 19 (76.0%) completed treatment through week 24 and 6 (24.0%) discontinued the study agent early, with 5 of the 6 discontinuing due to adverse events. At week 24, the median percent change in proteinuria from baseline to week 24 in sirukumab-treated patients was 0.0% (95% confidence interval -61.8, 39.6). In contrast, the 4 placebo-treated patients showed an increase in proteinuria (median percent reduction -43.3%) at week 24. Of note, a subset of 5 sirukumab-treated patients had ≥50% improvement in their P:C ratio through week 28. In the sirukumab group, 47.6% of patients experienced ≥1 serious adverse event through week 40; most were infection-related. No deaths or malignancies occurred. No serious adverse events were observed in the 4 placebo-treated patients.
Conclusion: This proof-of-concept study did not demonstrate the anticipated efficacy nor did it demonstrate an acceptable safety profile for sirukumab treatment in this population of patients with active LN receiving concomitant immunosuppressive treatment.
Trial registration: ClinicalTrials.gov NCT01273389.
© 2016 The Authors. Arthritis & Rheumatology published by Wiley Periodicals, Inc. on behalf of the American College of Rheumatology.
Figures

References
-
- Rovin BH, Stillman IE. The kidney in systemic lupus erythematosus In: Lahita RG, editor. Systemic lupus erythematosus. Fifth ed. London: Academic Press; 2011. p. 769–814.
-
- Cervera R, Khamashta MA, Font J, Sebastiani GD, Gil A, Lavilla P, et al, and the European Working Party on Systemic Lupus Erythematosus . Morbidity and mortality in systemic lupus erythematosus during a 5‐year period: a multicenter prospective study of 1,000 patients. Medicine (Baltimore) 1999;78:167–75. - PubMed
-
- Mok CC, Kwok RC, Yip PS. Effect of renal disease on the standardized mortality ratio and life expectancy of patients with systemic lupus erythematosus. Arthritis Rheum 2013;65:2154–60. - PubMed
-
- Furie R, Nicholls K, Cheng TT, Houssiau F, Burgos‐Vargas R, Chen SL, et al. Efficacy and safety of abatacept in lupus nephritis: a twelve‐month, randomized, double‐blind study. Arthritis Rheumatol 2014;66:379–89. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

