A Phase I Study of Unimolecular Pentavalent (Globo-H-GM2-sTn-TF-Tn) Immunization of Patients with Epithelial Ovarian, Fallopian Tube, or Peritoneal Cancer in First Remission
- PMID: 27110823
- PMCID: PMC4846855
- DOI: 10.3390/cancers8040046
A Phase I Study of Unimolecular Pentavalent (Globo-H-GM2-sTn-TF-Tn) Immunization of Patients with Epithelial Ovarian, Fallopian Tube, or Peritoneal Cancer in First Remission
Abstract
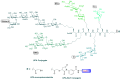
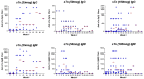
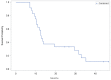
We conducted a phase I study in ovarian cancer patients to evaluate the safety and immunogenicity of a synthetic unimolecular pentavalent carbohydrate vaccine (Globo-H, GM2, sTn, TF, and Tn) supported on a peptide backbone, conjugated to keyhole limpet haemocyanin (KLH), and mixed with immunological adjuvant QS-21. Twenty-four advanced-stage, poor-risk, first-remission ovarian cancer patients were enrolled from January 2011-Septermber 2013. Three dose levels were planned (25, 50, 100 mcg) with three cohorts of six patients each, with an additional 6-patient expansion cohort at the MTD. ELISA serologic IgM and IgG responses for each antigen was defined as positive response if antibody titers were ≥1:80 over the respective patient's pre-vaccination serum. The study would be considered positive if at least four of 12 patients treated at the MTD showed immune responses for at least three of the five antigens. Twenty-four patients (median age, 54 years [range, 36-68]) were included in the safety analysis. Histology was high-grade serous in 22 patients (92%); 18 had stage III and six stage IV disease. The vaccine was well-tolerated at all doses, with no DLTs. At the highest treated dose, IgG and/or IgM responses were recorded against ≥3 antigens in 9/12 patients (75%), ≥4 in 7/12 (58%), and 5 in 3/12 (25%). With a median follow-up of 19 months (range, 2-39), 20 patients (83%) recurred and six (25%) died. The unimolecular pentavalent vaccine construct was shown to be safe and immunogenic. Such a construct greatly simplifies regulatory requirements and manufacturing, facilitates scalability, and provides adaptability.
Keywords: QS-21; immunogenicity; ovarian cancer; peptide vaccine; remission; unimolecular vaccine.
Figures
References
-
- Markman M., Markman J., Webster K., Zanotti K., Kulp B., Peterson G., Belinson J. Duration of response to second-line, platinum-based chemotherapy for ovarian cancer: Implications for patient management and clinical trial design. J. Clin. Oncol. 2004;22:3120–3125. doi: 10.1200/JCO.2004.05.195. - DOI - PubMed
-
- Syrios J., Banerjee S., Kaye S.B. Advanced epithelial ovarian cancer: From standard chemotherapy to promising molecular pathway targets—where are we now? Anticancer Res. 2014;34:2069–2077. - PubMed
-
- Zhang S., Zhang H.S., Cordon-Cardo C., Ragupathi G., Livingston P.O. Selection of tumor antigens as targets for immune attack using immunohistochemistry: Protein antigens. Clin. Cancer Res. 1998;4:2669–2676. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous