Brief Report: Estimating Disease Activity Using Multi-Biomarker Disease Activity Scores in Rheumatoid Arthritis Patients Treated With Abatacept or Adalimumab
- PMID: 27111089
- PMCID: PMC6099512
- DOI: 10.1002/art.39714
Brief Report: Estimating Disease Activity Using Multi-Biomarker Disease Activity Scores in Rheumatoid Arthritis Patients Treated With Abatacept or Adalimumab
Abstract
Objective: To assess the ability of a multi-biomarker disease activity (MBDA) test (Vectra DA) to reflect clinical measures of disease activity in patients enrolled in the AMPLE (Abatacept Versus Adalimumab Comparison in Biologic-Naive RA Subjects with Background Methotrexate) trial.
Methods: In the AMPLE trial, patients with active rheumatoid arthritis (RA) who were naive to biologic agents and had an inadequate response to methotrexate were randomized (1:1) to receive subcutaneous abatacept (125 mg every week) or subcutaneous adalimumab (40 mg every 2 weeks), with background methotrexate, for 2 years. The MBDA score was determined using serum samples collected at baseline, month 3, and years 1 and 2. The adjusted mean change from baseline in the MBDA score was compared between the abatacept and adalimumab treatment groups. Cross-tabulation was used to compare the MBDA score with the following clinical measures of disease activity: Clinical Disease Activity Index (CDAI), Simplified Disease Activity Index (SDAI), Disease Activity Score in 28 joints using the C-reactive protein level (DAS28-CRP), and Routine Assessment of Patient Index Data 3 (RAPID-3).
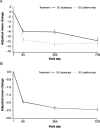
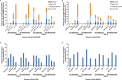
Results: In total, 318 patients were randomized to receive abatacept, and 328 were randomized to receive adalimumab; MBDA data were available for 259 and 265 patients, respectively. No association between the MBDA score and disease activity defined by the CDAI, SDAI, DAS28-CRP, or RAPID-3 in the abatacept and adalimumab treatment groups was observed.
Conclusion: The MBDA score did not reflect clinical disease activity in patients enrolled in AMPLE and should not be used to guide decision-making in the management of RA, particularly for patients who receive abatacept or adalimumab as the first biologic agent.
Trial registration: ClinicalTrials.gov NCT00929864.
© 2016, American College of Rheumatology.
Figures
Comment in
-
Editorial: The Multi-Biomarker Disease Activity Test for Rheumatoid Arthritis: Is It a Valid Measure of Disease Activity?Arthritis Rheumatol. 2016 Sep;68(9):2061-6. doi: 10.1002/art.39716. Arthritis Rheumatol. 2016. PMID: 27111349 No abstract available.
-
Reanalysis of the Multi-Biomarker Disease Activity Score for Assessing Disease Activity in the Abatacept Versus Adalimumab Comparison in Biologic-Naive Rheumatoid Arthritis Subjects with Background Methotrexate Study: Comment on the Article by Fleischmann et al.Arthritis Rheumatol. 2017 Apr;69(4):863-865. doi: 10.1002/art.39981. Epub 2017 Mar 3. Arthritis Rheumatol. 2017. PMID: 27813312 Free PMC article. No abstract available.
-
Limited Value of the Multi-Biomarker Disease Activity Assay Compared to the Routine Assessment of Patient Index Data 3 (RAPID3) Score in the Prognosis of Important Clinical Outcomes in Rheumatoid Arthritis: Comment on the Article by Fleischmann et al and Accompanying Editorial by Davis.Arthritis Rheumatol. 2017 Apr;69(4):866-867. doi: 10.1002/art.40022. Epub 2017 Mar 3. Arthritis Rheumatol. 2017. PMID: 27992686 No abstract available.
-
Reply.Arthritis Rheumatol. 2017 Apr;69(4):867-868. doi: 10.1002/art.40021. Epub 2017 Feb 28. Arthritis Rheumatol. 2017. PMID: 27992708 No abstract available.
References
-
- Singh JA, Saag KG, Bridges SL Jr, Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2016;68:1–25. - PubMed
-
- Smolen JS, Breedveld FC, Schiff MH, Kalden JR, Emery P, Eberl G, et al. A Simplified Disease Activity Index for rheumatoid arthritis for use in clinical practice. Rheumatology (Oxford) 2003;42:244–57. - PubMed
-
- Prevoo ML, van 't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty‐eight–joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

