Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics
- PMID: 27111232
- PMCID: PMC4887186
- DOI: 10.1172/JCI86062
Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics
Abstract
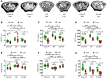
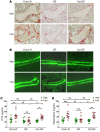
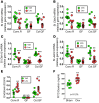
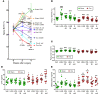
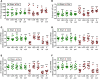
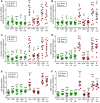
A eubiotic microbiota influences many physiological processes in the metazoan host, including development and intestinal homeostasis. Here, we have shown that the intestinal microbiota modulates inflammatory responses caused by sex steroid deficiency, leading to trabecular bone loss. In murine models, sex steroid deficiency increased gut permeability, expanded Th17 cells, and upregulated the osteoclastogenic cytokines TNFα (TNF), RANKL, and IL-17 in the small intestine and the BM. In germ-free (GF) mice, sex steroid deficiency failed to increase osteoclastogenic cytokine production, stimulate bone resorption, and cause trabecular bone loss, demonstrating that the gut microbiota is central in sex steroid deficiency-induced trabecular bone loss. Furthermore, we demonstrated that twice-weekly treatment of sex steroid-deficient mice with the probiotics Lactobacillus rhamnosus GG (LGG) or the commercially available probiotic supplement VSL#3 reduces gut permeability, dampens intestinal and BM inflammation, and completely protects against bone loss. In contrast, supplementation with a nonprobiotic strain of E. coli or a mutant LGG was not protective. Together, these data highlight the role that the gut luminal microbiota and increased gut permeability play in triggering inflammatory pathways that are critical for inducing bone loss in sex steroid-deficient mice. Our data further suggest that probiotics that decrease gut permeability have potential as a therapeutic strategy for postmenopausal osteoporosis.
Figures

Comment in
-
From the gut to the strut: where inflammation reigns, bone abstains.J Clin Invest. 2016 Jun 1;126(6):2045-8. doi: 10.1172/JCI87430. Epub 2016 Apr 25. J Clin Invest. 2016. PMID: 27111233 Free PMC article.

