Comparing the Clinical Outcomes between Drug Eluting Stents and Bare Metal Stents in Patients with Insulin-Treated Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of 10 Randomized Controlled Trials
- PMID: 27111304
- PMCID: PMC4844102
- DOI: 10.1371/journal.pone.0154064
Comparing the Clinical Outcomes between Drug Eluting Stents and Bare Metal Stents in Patients with Insulin-Treated Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of 10 Randomized Controlled Trials
Abstract
Background: Several studies have shown Drug Eluting Stents (DES) to be better compared to Bare Metal Stents (BMS) in patients with type 2 Diabetes Mellitus (T2DM). Since, the adverse clinical outcomes in patients with Insulin-Treated Type 2 Diabetes Mellitus (ITDM) implanted with DES and BMS have not been previously studied, we aim to compare the clinical outcomes in similar patients with cardiovascular diseases, treated with DES and BMS.
Methods: Randomized Controlled Trials (RCTs) comparing patients treated with DES and BMS were searched from PubMed and EMBASE databases. Outcome data for the patients with ITDM were carefully extracted. Major Adverse Cardiac Events (MACEs), mortality, Target Vessel Revascularization (TVR), Target Lesion Revascularization (TLR), Myocardial Infarction (MI) and Stent Thrombosis (ST) were considered as the clinical endpoints for this analysis. Odds ratios (OR) with 95% confidence intervals (CIs) were calculated and the pooled analyses were performed with RevMan 5.3 software.
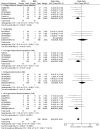
Results: Ten RCTs consisting of 830 patients with ITDM (477 patients in the DES group and 353 patients in the BMS group) from a total number of 9,141 patients were included in this analysis. During a follow-up period from one month to one year, MACEs were not increased with the use of DES in these patients with ITDM. At 9 months, MACEs were significantly lower in the DES group with OR: 0.40, 95% CI: 0.23-0.72; P = 0.002 with no increase in mortality. TVR and TLR also favored the DES group with OR: 0.44, 95% CI: 0.22-0.88, P = 0.02 and OR: 0.28, 95% CI: 0.14-0.53; P = 0.0001 respectively at 9 months, and OR: 0.46, 95% CI: 0.23-0.94, P = 0.03 and OR: 0.28, 95% CI: 0.14-0.55; P = 0.0003 respectively at one year. Results for MI, and ST were not statistically significant.
Conclusion: Compared to BMS, DES were associated with a significantly lower rate of repeated revascularization, without any increase in MACEs or mortality in these patients with ITDM during a follow up period of one year. However, due to the very small population size, further studies with a larger number of randomized patients are required to completely solve this issue.
Conflict of interest statement
Figures




References
-
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004; 27:1047–1053. - PubMed
-
- Kugelmass AD, Cohen DJ, Houser F, Mack M, Simon AW, Battaglia SL, et al. The influence of diabetes mellitus on the practice and outcomes of percutaneous coronary intervention in thecommunity: a report from the HCA database. J Invasive Cardiol. 2003; 15(10):568–74. - PubMed
-
- Akin I, Bufe A, Schneider S, Reinecke H, Eckardt L, Richardt G, et al. Clinical outcomes in diabetic and non-diabetic patients with drug-eluting stents: results from the first phase of the prospective multicenter German DES.DE registry. Clin Res Cardiol. 2010; 99(6):393–400. 10.1007/s00392-010-0136-8 - DOI - PubMed
-
- Iijima R, Byrne RA, Dibra A, Ndrepepa G, Spaulding C, Laarman GJ, et al. Drug-eluting stents versus bare-metal stents in diabetic patients with ST-segment elevation acute myocardial infarction: a pooled analysis of individual patient data from seven randomized trials. Rev Esp Cardiol. 2009; 62(4):354–64. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

