EEG desynchronization during phasic REM sleep suppresses interictal epileptic activity in humans
- PMID: 27112123
- PMCID: PMC4949560
- DOI: 10.1111/epi.13389
EEG desynchronization during phasic REM sleep suppresses interictal epileptic activity in humans
Abstract
Objective: Rapid eye movement (REM) sleep has a suppressing effect on epileptic activity. This effect might be directly related to neuronal desynchronization mediated by cholinergic neurotransmission. We investigated whether interictal epileptiform discharges (IEDs) and high frequency oscillations-a biomarker of the epileptogenic zone-are evenly distributed across phasic and tonic REM sleep. We hypothesized that IEDs are more suppressed during phasic REM sleep because of additional cholinergic drive.
Methods: Twelve patients underwent polysomnography during long-term combined scalp-intracerebral electroencephalography (EEG) recording. After sleep staging in the scalp EEG, we identified segments of REM sleep with rapid eye movements (phasic REM) and segments of REM sleep without rapid eye movements (tonic REM). In the intracerebral EEG, we computed the power in frequencies <30 Hz and from 30 to 500 Hz, and marked IEDs, ripples (>80 Hz) and fast ripples (>250 Hz). We grouped the intracerebral channels into channels in the seizure-onset zone (SOZ), the exclusively irritative zone (EIZ), and the normal zone (NoZ).
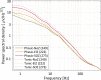
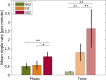
Results: Power in frequencies <30 Hz was lower during phasic than tonic REM sleep (p < 0.001), most likely reflecting increased desynchronization. IEDs, ripples and fast ripples, were less frequent during phasic than tonic REM sleep (phasic REM sleep: 39% of spikes, 35% of ripples, 18% of fast ripples, tonic REM sleep: 61% of spikes, 65% of ripples, 82% of fast ripples; p < 0.001). In contrast to ripples in the epileptogenic zone, physiologic ripples were more abundant during phasic REM sleep (phasic REM sleep: 73% in NoZ, 30% in EIZ, 28% in SOZ, tonic REM sleep: 27% in NoZ, 70% in EIZ, 72% in SOZ; p < 0.001).
Significance: Phasic REM sleep has an enhanced suppressive effect on IEDs, corroborating the role of EEG desynchronization in the suppression of interictal epileptic activity. In contrast, physiologic ripples were increased during phasic REM sleep, possibly reflecting REM-related memory consolidation and dreaming.
Keywords: Epilepsy; High-frequency oscillations; Intracerebral EEG; Polysomnography; Sleep.
Wiley Periodicals, Inc. © 2016 The Authors. Epilepsia published by Wiley Periodicals, Inc. on behalf of International League Against Epilepsy.
Figures



References
-
- Lieb JP, Joseph JP, Engel J Jr, et al. Sleep state and seizure foci related to depth spike activity in patients with temporal lobe epilepsy. Electroencephalogr Clin Neurophysiol 1980;49:538–557. - PubMed
-
- Rossi GF, Colicchio G, Pola P. Interictal epileptic activity during sleep: a stereo‐EEG study in patients with partial epilepsy. Electroencephalogr Clin Neurophysiol 1984;58:97–106. - PubMed
-
- Montplaisir J, Laverdière M, Saint‐Hilaire JM, et al. Nocturnal sleep recording in partial epilepsy: a study with depth electrodes. J Clin Neurophysiol 1987;4:383–388. - PubMed
-
- Sammaritano M, Gigli GL, Gotman J. Interictal spiking during wakefulness and sleep and the localization of foci in temporal lobe epilepsy. Neurology 1991;41:290–297. - PubMed
-
- Autret A, Lucas B, Hommet C, et al. Sleep and the epilepsies. J Neurol 1997;244:S10–S17. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

