Using magnetic resonance imaging to assess visual deficits: a review
- PMID: 27112223
- PMCID: PMC4855621
- DOI: 10.1111/opo.12293
Using magnetic resonance imaging to assess visual deficits: a review
Abstract
Purpose: Over the last two decades, magnetic resonance imaging (MRI) has been widely used in neuroscience research to assess both structure and function in the brain in health and disease. With regard to vision research, prior to the advent of MRI, researchers relied on animal physiology and human post-mortem work to assess the impact of eye disease on visual cortex and connecting structures. Using MRI, researchers can non-invasively examine the effects of eye disease on the whole visual pathway, including the lateral geniculate nucleus, striate and extrastriate cortex. This review aims to summarise research using MRI to investigate structural, chemical and functional effects of eye diseases, including: macular degeneration, retinitis pigmentosa, glaucoma, albinism, and amblyopia.
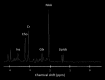
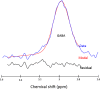
Recent findings: Structural MRI has demonstrated significant abnormalities within both grey and white matter densities across both visual and non-visual areas. Functional MRI studies have also provided extensive evidence of functional changes throughout the whole of the visual pathway following visual loss, particularly in amblyopia. MR spectroscopy techniques have also revealed several abnormalities in metabolite concentrations in both glaucoma and age-related macular degeneration. GABA-edited MR spectroscopy on the other hand has identified possible evidence of plasticity within visual cortex.
Summary: Collectively, using MRI to investigate the effects on the visual pathway following disease and dysfunction has revealed a rich pattern of results allowing for better characterisation of disease. In the future MRI will likely play an important role in assessing the impact of eye disease on the visual pathway and how it progresses over time.
Keywords: albinism; amblyopia; anophthalmia; functional magnetic resonance imaging; glaucoma; macular degeneration; magnetic resonance imaging; magnetic resonance spectroscopy; ophthalmology; visual deficit.
© 2016 The The Authors Ophthalmic and Physiological Optics published by John Wiley & Sons Ltd on behalf of College of Optometrists.
Figures


References
-
- Wandell BA, Dumoulin SO & Brewer AA. Visual field maps in human cortex. Neuron 2007; 56: 366–383. - PubMed
-
- Ragge N, Bridge H, Alexander I et al Investigating developmental plasticity in human anophthalmia. Invest Ophthalmol Vis Sci 2008;49(ARVO abstract):4501.
-
- Shaw GM, Carmichael SL, Yang W, Harris JA, Finnell RH & Lammer EJ. Epidemiologic characteristics of anophthalmia and bilateral microphthalmia among 2.5 million births in California, 1989–1997. Am J Med Genet A 2005; 137: 36–40. - PubMed
-
- Bridge H, Cowey A, Ragge N & Watkins K. Imaging studies in congenital anophthalmia reveal preservation of brain architecture in “visual” cortex. Brain 2009; 132(Pt 12): 3467–3480. - PubMed
-
- Lim LS, Mitchell P, Seddon JM, Holz FG & Wong TY. Age‐related macular degeneration. Lancet 2012; 379: 1728–1738. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

