Infrared reflectance imaging in age-related macular degeneration
- PMID: 27112225
- PMCID: PMC5347934
- DOI: 10.1111/opo.12283
Infrared reflectance imaging in age-related macular degeneration
Abstract
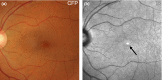
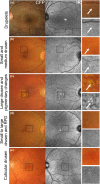
Purpose: The purpose of this article is to describe the appearance of age-related macular degeneration (AMD) phenotypes using infrared (IR) reflectance imaging. IR reflectance imaging of the retina has the potential to highlight specific sub-retinal features and pathology. However, its role in macular disease, specifically AMD, is often underestimated and requires clarification.
Recent findings: Recent advances in clinical methods, imaging and scientific knowledge may be integrated to improve the accuracy of disease stratification in AMD. In particular, IR imaging holds an underutilised sensitivity to detect reticular pseudodrusen, which have been repeatedly described as a high-risk sign for late AMD.
Summary: This article provides clinically relevant descriptions of AMD phenotypes using IR reflectance imaging. The findings are integrated with images from cases seen at the Centre for Eye Health. As primary eye-care providers assume a critical role in the detection, diagnosis and management of AMD, we also provide a chair-side reference to assist clinicians in interpreting IR images in AMD.
Keywords: age-related macular degeneration; chair-side reference; drusen; infrared; retina.
© 2016 The Authors Ophthalmic and Physiological Optics published by John Wiley & Sons Ltd on behalf of College of Optometrists.
Figures


References
-
- Bourne RR, Stevens GA, White RA et al Causes of vision loss worldwide, 1990–2010: a systematic analysis. Lancet Glob Health 2013; 1: e339–e349. - PubMed
-
- Wong WL, Su X, Li X et al Global prevalence of age‐related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta‐analysis. Lancet Glob Health 2014; 2: e106–e116. - PubMed
-
- Chong GT & Lee RK. Glaucoma versus red disease: imaging and glaucoma diagnosis. Curr Opin Ophthalmol 2012; 23: 79–88. - PubMed
-
- Pecen PE & Kaiser PK. Current phase 1/2 research for neovascular age‐related macular degeneration. Curr Opin Ophthalmol 2015; 26: 188–193. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

