Cacna1g is a genetic modifier of epilepsy caused by mutation of voltage-gated sodium channel Scn2a
- PMID: 27112236
- PMCID: PMC4985168
- DOI: 10.1111/epi.13390
Cacna1g is a genetic modifier of epilepsy caused by mutation of voltage-gated sodium channel Scn2a
Abstract
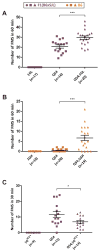
More than 1,200 mutations in neuronal voltage-gated sodium channel (VGSC) genes have been identified in patients with several epilepsy syndromes. A common feature of genetic epilepsies is variable expressivity among individuals with the same mutation. The Scn2a(Q54) transgenic mouse model has a mutation in Scn2a that results in spontaneous epilepsy. Scn2a(Q54) phenotype severity varies depending on the genetic strain background, making it a useful model for identifying and characterizing epilepsy modifier genes. Scn2a(Q54) mice on the [C57BL/6JxSJL/J]F1 background exhibit earlier seizure onset, elevated spontaneous seizure frequency, and decreased survival compared to Scn2a(Q54) mice congenic on the C57BL/6J strain. Genetic mapping and RNA-Seq analysis identified Cacna1g as a candidate modifier gene at the Moe1 locus, which influences Scn2a(Q54) phenotype severity. In this study, we evaluated the modifier potential of Cacna1g, encoding the Cav3.1 voltage-gated calcium channel, by testing whether transgenic alteration of Cacna1g expression modifies severity of the Scn2a(Q54) seizure phenotype. Scn2a(Q54) mice exhibited increased spontaneous seizure frequency with elevated Cacna1g expression and decreased seizure frequency with decreased Cacna1g expression. These results provide support for Cacna1g as an epilepsy modifier gene and suggest that modulation of Cav3.1 may be an effective therapeutic strategy.
Keywords: Genetics; Mouse model; Seizures; Voltage-gated calcium channels; Voltage-gated ion channels.
Wiley Periodicals, Inc. © 2016 International League Against Epilepsy.
Conflict of interest statement
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Figures

References
-
- Kearney JA, Plummer NW, Smith MR, et al. A gain-of-function mutation in the sodium channel gene Scn2a results in seizures and behavioral abnormalities. Neuroscience. 2001;102:307–317. - PubMed
-
- Bergren SK, Chen S, Galecki A, et al. Genetic modifiers affecting severity of epilepsy caused by mutation of sodium channel Scn2a. Mamm Genome. 2005;16:683–690. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

