The Staphylococcus aureus polysaccharide capsule and Efb-dependent fibrinogen shield act in concert to protect against phagocytosis
- PMID: 27112346
- PMCID: PMC4977062
- DOI: 10.1099/mic.0.000293
The Staphylococcus aureus polysaccharide capsule and Efb-dependent fibrinogen shield act in concert to protect against phagocytosis
Abstract
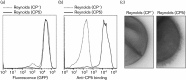
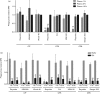
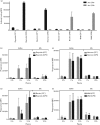
Staphylococcus aureus has developed many mechanisms to escape from human immune responses. To resist phagocytic clearance, S. aureus expresses a polysaccharide capsule, which effectively masks the bacterial surface and surface-associated proteins, such as opsonins, from recognition by phagocytic cells. Additionally, secretion of the extracellular fibrinogen binding protein (Efb) potently blocks phagocytic uptake of the pathogen. Efb creates a fibrinogen shield surrounding the bacteria by simultaneously binding complement C3b and fibrinogen at the bacterial surface. By means of neutrophil phagocytosis assays with fluorescently labelled encapsulated serotype 5 (CP5) and serotype 8 (CP8) strains we compare the immune-modulating function of these shielding mechanisms. The data indicate that, in highly encapsulated S. aureus strains, the polysaccharide capsule is able to prevent phagocytic uptake at plasma concentrations <10 %, but loses its protective ability at higher concentrations of plasma. Interestingly, Efb shows a strong inhibitory effect on both capsule-negative and encapsulated strains at all tested plasma concentrations. Furthermore, the results suggest that both shielding mechanisms can exist simultaneously and collaborate to provide optimal protection against phagocytosis at a broad range of plasma concentrations. As opsonizing antibodies will be shielded from recognition by either mechanism, incorporating both capsular polysaccharides and Efb in future vaccines could be of great importance.
Figures


Similar articles
-
Phagocytosis escape by a Staphylococcus aureus protein that connects complement and coagulation proteins at the bacterial surface.PLoS Pathog. 2013;9(12):e1003816. doi: 10.1371/journal.ppat.1003816. Epub 2013 Dec 12. PLoS Pathog. 2013. PMID: 24348255 Free PMC article.
-
Staphylococcal protein Ecb impairs complement receptor-1 mediated recognition of opsonized bacteria.PLoS One. 2017 Mar 8;12(3):e0172675. doi: 10.1371/journal.pone.0172675. eCollection 2017. PLoS One. 2017. PMID: 28273167 Free PMC article.
-
Antibodies to Staphylococcus aureus capsular polysaccharides 5 and 8 perform similarly in vitro but are functionally distinct in vivo.Virulence. 2017 Aug 18;8(6):859-874. doi: 10.1080/21505594.2016.1270494. Epub 2016 Dec 9. Virulence. 2017. PMID: 27936346 Free PMC article.
-
The staphylococcal surface-glycopolymer wall teichoic acid (WTA) is crucial for complement activation and immunological defense against Staphylococcus aureus infection.Immunobiology. 2016 Oct;221(10):1091-101. doi: 10.1016/j.imbio.2016.06.003. Epub 2016 Jun 15. Immunobiology. 2016. PMID: 27424796 Review.
-
Staphylococcus aureus capsular polysaccharides.Clin Microbiol Rev. 2004 Jan;17(1):218-34. doi: 10.1128/CMR.17.1.218-234.2004. Clin Microbiol Rev. 2004. PMID: 14726462 Free PMC article. Review.
Cited by
-
Comparative Transcriptomic Analysis of Staphylococcus aureus Associated with Periprosthetic Joint Infection under in Vivo and in Vitro Conditions.J Mol Diagn. 2021 Aug;23(8):986-999. doi: 10.1016/j.jmoldx.2021.05.011. Epub 2021 Jun 5. J Mol Diagn. 2021. PMID: 34098085 Free PMC article.
-
Host-Pathogen Interface: Progress in Understanding the Pathogenesis of Infection Due to Multidrug-Resistant Bacteria in the Intensive Care Unit.J Infect Dis. 2017 Feb 15;215(suppl_1):S1-S8. doi: 10.1093/infdis/jiw405. J Infect Dis. 2017. PMID: 28375516 Free PMC article.
-
Recognition of Staphylococcus aureus by the pattern recognition molecules langerin, mannan-binding lectin, and surfactant protein D: the influence of capsular polysaccharides and wall teichoic acid.Front Immunol. 2025 Jan 7;15:1504886. doi: 10.3389/fimmu.2024.1504886. eCollection 2024. Front Immunol. 2025. PMID: 39850879 Free PMC article.
-
BBK32 attenuates antibody-dependent complement-mediated killing of infectious Borreliella burgdorferi isolates.PLoS Pathog. 2025 Jul 24;21(7):e1013361. doi: 10.1371/journal.ppat.1013361. eCollection 2025 Jul. PLoS Pathog. 2025. PMID: 40705830 Free PMC article.
-
Dendritic Cells Internalize Staphylococcus aureus More Efficiently than Staphylococcus epidermidis, but Do Not Differ in Induction of Antigen-Specific T Cell Proliferation.Microorganisms. 2019 Dec 20;8(1):19. doi: 10.3390/microorganisms8010019. Microorganisms. 2019. PMID: 31861881 Free PMC article.
References
-
- Boyle-Vavra S., Li X., Alam M. T., Read T. D., Sieth J., Cywes-Bentley C., Dobbins G., David M. Z., Kumar N., et al. (2015). USA300 and USA500 clonal lineages of Staphylococcus aureus do not produce a capsular polysaccharide due to conserved mutations in the cap5 locus. MBio 61–10.10.1128/mBio.02585-14 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

