Substituted quinolines as noncovalent proteasome inhibitors
- PMID: 27112450
- PMCID: PMC5724766
- DOI: 10.1016/j.bmc.2016.04.005
Substituted quinolines as noncovalent proteasome inhibitors
Abstract
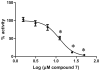
Screening of a library of diverse heterocyclic scaffolds identified substituted quinolines as inhibitors of the human proteasome. The heterocyclic library was prepared via a novel titanium-catalyzed multicomponent coupling reaction, which rendered a diverse set of isoxazoles, pyrimidines, pyrroles, pyrazoles and quinolines. SAR of the parent lead compound indicated that hydrophobic residues on the benzo-moiety significantly improved potency. Lead compound 25 inhibits the chymotryptic-like proteolytic activity of the proteasome (IC50 5.4μM), representing a new class of nonpeptidic, noncovalent proteasome inhibitors.
Keywords: Inhibitors; Noncovalent; Proteasome; Quinolines.
Copyright © 2016. Published by Elsevier Ltd.
Figures


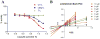
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Miscellaneous

