Oxytocin Enhances Social Recognition by Modulating Cortical Control of Early Olfactory Processing
- PMID: 27112498
- PMCID: PMC4860033
- DOI: 10.1016/j.neuron.2016.03.033
Oxytocin Enhances Social Recognition by Modulating Cortical Control of Early Olfactory Processing
Abstract
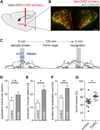
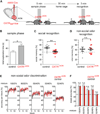
Oxytocin promotes social interactions and recognition of conspecifics that rely on olfaction in most species. The circuit mechanisms through which oxytocin modifies olfactory processing are incompletely understood. Here, we observed that optogenetically induced oxytocin release enhanced olfactory exploration and same-sex recognition of adult rats. Consistent with oxytocin's function in the anterior olfactory cortex, particularly in social cue processing, region-selective receptor deletion impaired social recognition but left odor discrimination and recognition intact outside a social context. Oxytocin transiently increased the drive of the anterior olfactory cortex projecting to olfactory bulb interneurons. Cortical top-down recruitment of interneurons dynamically enhanced the inhibitory input to olfactory bulb projection neurons and increased the signal-to-noise of their output. In summary, oxytocin generates states for optimized information extraction in an early cortical top-down network that is required for social interactions with potential implications for sensory processing deficits in autism spectrum disorders.
Keywords: anterior olfactory nucleus; centrifugal; granule cells; mitral cells.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
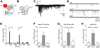





Comment in
-
Sniffing and Oxytocin: Effects on Olfactory Memories.Neuron. 2016 May 4;90(3):431-3. doi: 10.1016/j.neuron.2016.04.033. Neuron. 2016. PMID: 27151635
Similar articles
-
Oxytocin and Olfaction.Curr Top Behav Neurosci. 2018;35:55-75. doi: 10.1007/7854_2017_8. Curr Top Behav Neurosci. 2018. PMID: 28812265 Review.
-
Vaginocervical stimulation enhances social recognition memory in rats via oxytocin release in the olfactory bulb.Neuroscience. 2008 Mar 27;152(3):585-93. doi: 10.1016/j.neuroscience.2008.01.024. Epub 2008 Jan 25. Neuroscience. 2008. PMID: 18304743
-
Experience-Dependent Plasticity in Accessory Olfactory Bulb Interneurons following Male-Male Social Interaction.J Neurosci. 2017 Jul 26;37(30):7240-7252. doi: 10.1523/JNEUROSCI.1031-17.2017. Epub 2017 Jun 28. J Neurosci. 2017. PMID: 28659282 Free PMC article.
-
A Computational Model of Oxytocin Modulation of Olfactory Recognition Memory.eNeuro. 2019 Aug 29;6(4):ENEURO.0201-19.2019. doi: 10.1523/ENEURO.0201-19.2019. Print 2019 Jul/Aug. eNeuro. 2019. PMID: 31399493 Free PMC article.
-
Centrifugal drive onto local inhibitory interneurons of the olfactory bulb.Ann N Y Acad Sci. 2009 Jul;1170:239-54. doi: 10.1111/j.1749-6632.2009.03913.x. Ann N Y Acad Sci. 2009. PMID: 19686142 Review.
Cited by
-
Age-related decline in social interaction is associated with decreased c-Fos induction in select brain regions independent of oxytocin receptor expression profiles.Aging Brain. 2024 Jan 23;5:100107. doi: 10.1016/j.nbas.2024.100107. eCollection 2024. Aging Brain. 2024. PMID: 38313579 Free PMC article.
-
Vasopressin receptor 1a, oxytocin receptor, and oxytocin knockout male and female mice display normal perceptual abilities towards non-social odorants.Horm Behav. 2023 Feb;148:105302. doi: 10.1016/j.yhbeh.2022.105302. Epub 2023 Jan 9. Horm Behav. 2023. PMID: 36628861 Free PMC article.
-
Amylin-Calcitonin receptor signaling in the medial preoptic area mediates affiliative social behaviors in female mice.Nat Commun. 2022 Feb 8;13(1):709. doi: 10.1038/s41467-022-28131-z. Nat Commun. 2022. PMID: 35136064 Free PMC article.
-
The neural mechanisms and circuitry of the pair bond.Nat Rev Neurosci. 2018 Nov;19(11):643-654. doi: 10.1038/s41583-018-0072-6. Nat Rev Neurosci. 2018. PMID: 30301953 Free PMC article. Review.
-
Oxytocin enhances neural approach towards social and non-social stimuli of high personal relevance.Sci Rep. 2021 Dec 8;11(1):23589. doi: 10.1038/s41598-021-02914-8. Sci Rep. 2021. PMID: 34880300 Free PMC article. Clinical Trial.
References
-
- Alonso M, Lepousez G, Sebastien W, Bardy C, Gabellec MM, Torquet N, Lledo PM. Activation of adult-born neurons facilitates learning and memory. Nat. Neurosci. 2012;15:897–904. - PubMed
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth. American Psychiatric Association; 2013.
-
- Asperger H. Die autistischen psychopathen im kindesalter. Arch. Psychiatr. Nervenkr. 1944;177:76–136.
-
- Balu R, Strowbridge BW. Opposing inward and outward conductances regulate rebound discharges in olfactory mitral cells. J. Neurophysiol. 2007;97:1959–1968. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

