Regulatory T cell expressed MyD88 is critical for prolongation of allograft survival
- PMID: 27112509
- PMCID: PMC5049733
- DOI: 10.1111/tri.12788
Regulatory T cell expressed MyD88 is critical for prolongation of allograft survival
Abstract
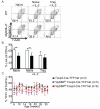
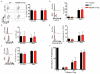
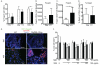
MyD88 signaling directly promotes T-cell survival and is required for optimal T-cell responses to pathogens. To examine the role of T-cell-intrinsic MyD88 signals in transplantation, we studied mice with targeted T-cell-specific MyD88 deletion. Contrary to expectations, we found that these mice were relatively resistant to prolongation of graft survival with anti-CD154 plus rapamycin in a class II-mismatched system. To specifically examine the role of MyD88 in Tregs, we created a Treg-specific MyD88-deficient mouse. Transplant studies in these animals replicated the findings observed with a global T-cell MyD88 knockout. Surprisingly, given the role of MyD88 in conventional T-cell survival, we found no defect in the survival of MyD88-deficient Tregs in vitro or in the transplant recipients and also observed intact cell homing and expression of Treg effector molecules. MyD88-deficient Tregs also fail to protect allogeneic bone marrow transplant recipients from chronic graft-versus-host disease, confirming the observations of defective regulation seen in a solid organ transplant system. Together, our data define MyD88 as having a divergent requirement for cell survival in non-Tregs and Tregs, and a yet-to-be defined survival-independent requirement for Treg function during the response to alloantigen.
Keywords: T cells; Treg; inflammation; transplantation.
© 2016 Steunstichting ESOT.
Figures

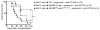
References
-
- Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, et al. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell. 1998;2(2):253–8. - PubMed
-
- Casanova JL, Abel L, Quintana-Murci L. Human TLRs and IL-1Rs in host defense: natural insights from evolutionary, epidemiological, and clinical genetics. Annu Rev Immunol. 2011;29:447–91. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

