Diverse human extracellular RNAs are widely detected in human plasma
- PMID: 27112789
- PMCID: PMC4853467
- DOI: 10.1038/ncomms11106
Diverse human extracellular RNAs are widely detected in human plasma
Erratum in
-
Corrigendum: Diverse human extracellular RNAs are widely detected in human plasma.Nat Commun. 2016 Jun 3;7:11902. doi: 10.1038/ncomms11902. Nat Commun. 2016. PMID: 27255613 Free PMC article. No abstract available.
Abstract
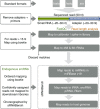
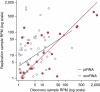
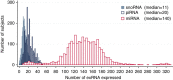
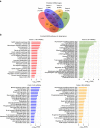
There is growing appreciation for the importance of non-protein-coding genes in development and disease. Although much is known about microRNAs, limitations in bioinformatic analyses of RNA sequencing have precluded broad assessment of other forms of small-RNAs in humans. By analysing sequencing data from plasma-derived RNA from 40 individuals, here we identified over a thousand human extracellular RNAs including microRNAs, piwi-interacting RNA (piRNA), and small nucleolar RNAs. Using a targeted quantitative PCR with reverse transcription approach in an additional 2,763 individuals, we characterized almost 500 of the most abundant extracellular transcripts including microRNAs, piRNAs and small nucleolar RNAs. The presence in plasma of many non-microRNA small-RNAs was confirmed in an independent cohort. We present comprehensive data to demonstrate the broad and consistent detection of diverse classes of circulating non-cellular small-RNAs from a large population.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- UH3 TR000921/TR/NCATS NIH HHS/United States
- UH2TR000921/TR/NCATS NIH HHS/United States
- UH2 TR000921/TR/NCATS NIH HHS/United States
- N01-HC 25195/HC/NHLBI NIH HHS/United States
- U54 HL112311/HL/NHLBI NIH HHS/United States
- U01 HL126495/HL/NHLBI NIH HHS/United States
- N01 HC025195/HL/NHLBI NIH HHS/United States
- U01HL126495/HL/NHLBI NIH HHS/United States
- UH3 TR000901/TR/NCATS NIH HHS/United States
- P01-HL085381/HL/NHLBI NIH HHS/United States
- UH2 TR000901/TR/NCATS NIH HHS/United States
- K23 HL127099/HL/NHLBI NIH HHS/United States
- UH2TR000901/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

