Size-tunable copper nanocluster aggregates and their application in hydrogen sulfide sensing on paper-based devices
- PMID: 27113330
- PMCID: PMC4845058
- DOI: 10.1038/srep24882
Size-tunable copper nanocluster aggregates and their application in hydrogen sulfide sensing on paper-based devices
Abstract
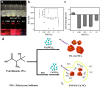
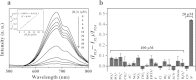
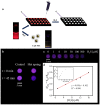
Polystyrene sulfonate (PSS), a strong polyelectrolyte, was used to prepare red photoluminescent PSS-penicillamine (PA) copper (Cu) nanoclusters (NC) aggregates, which displayed high selectivity and sensitivity to the detection of hydrogen sulfide (H2S). The size of the PSS-PA-Cu NC aggregates could be readily controlled from 5.5 μm to 173 nm using different concentrations of PSS, which enabled better dispersity and higher sensitivity towards H2S. PSS-PA-Cu NC aggregates provided rapid H2S detection by using the strong Cu-S interaction to quench NC photoluminescence as a sensing mechanism. As a result, a detection limit of 650 nM, which is lower than the maximum level permitted in drinking water by the World Health Organization, was achieved for the analysis of H2S in spring-water samples. Moreover, highly dispersed PSS-PA-Cu NC aggregates could be incorporated into a plate-format paper-based analytical device which enables ultra-low sample volumes (5 μL) and feature shorter analysis times (30 min) compared to conventional solution-based methods. The advantages of low reagent consumption, rapid result readout, limited equipment, and long-term storage make this platform sensitive and simple enough to use without specialized training in resource constrained settings.
Figures


Similar articles
-
Detection of hydrogen sulfide through photoluminescence quenching of penicillamine-copper nanocluster aggregates.Nanotechnology. 2014 May 16;25(19):195502. doi: 10.1088/0957-4484/25/19/195502. Epub 2014 Apr 24. Nanotechnology. 2014. PMID: 24762432
-
Polyamine-capped gold nanorod as a localized surface Plasmon resonance probe for rapid and sensitive copper(II) ion detection.J Colloid Interface Sci. 2015 Feb 1;439:7-11. doi: 10.1016/j.jcis.2014.10.023. Epub 2014 Oct 23. J Colloid Interface Sci. 2015. PMID: 25463169
-
A novel peptide-based fluorescence chemosensor for selective imaging of hydrogen sulfide both in living cells and zebrafish.Biosens Bioelectron. 2017 Jun 15;92:602-609. doi: 10.1016/j.bios.2016.10.050. Epub 2016 Oct 24. Biosens Bioelectron. 2017. PMID: 27829566
-
A polyoxometalate-based supramolecular chemosensor for rapid detection of hydrogen sulfide with dual signals.J Colloid Interface Sci. 2017 Jan 1;485:280-287. doi: 10.1016/j.jcis.2016.09.047. Epub 2016 Sep 22. J Colloid Interface Sci. 2017. PMID: 27684785
-
Fluorescence chemosensors for hydrogen sulfide detection in biological systems.Analyst. 2015 Mar 21;140(6):1772-86. doi: 10.1039/c4an01909a. Analyst. 2015. PMID: 25529122 Review.
Cited by
-
Antibacterial cellulose paper made with silver-coated gold nanoparticles.Sci Rep. 2017 Jun 9;7(1):3155. doi: 10.1038/s41598-017-03357-w. Sci Rep. 2017. PMID: 28600506 Free PMC article.
-
Temperature-dependent luminescent copper nanoclusters with noncovalent interactions for determination of β-galactosidase activity.Mikrochim Acta. 2024 Nov 28;191(12):768. doi: 10.1007/s00604-024-06844-w. Mikrochim Acta. 2024. PMID: 39607597
-
Review of Chemical Sensors for Hydrogen Sulfide Detection in Organisms and Living Cells.Sensors (Basel). 2023 Mar 21;23(6):3316. doi: 10.3390/s23063316. Sensors (Basel). 2023. PMID: 36992027 Free PMC article. Review.
-
Development of ratiometric sensing and white light-harvesting materials based on all-copper nanoclusters.Nanoscale Adv. 2018 Dec 3;1(3):1086-1095. doi: 10.1039/c8na00224j. eCollection 2019 Mar 12. Nanoscale Adv. 2018. PMID: 36133193 Free PMC article.
-
Development of a paper printed colorimetric sensor based on Cu-Curcumin nanoparticles for evolving point-of-care clinical diagnosis of sodium.Sci Rep. 2022 Apr 15;12(1):6247. doi: 10.1038/s41598-022-09852-z. Sci Rep. 2022. PMID: 35428770 Free PMC article.
References
-
- Chen P.-C., Yeh T.-Y., Ou C.-M., Shih C.-C. & Chang H.-T. Synthesis of aluminum oxide supported fluorescent gold nanodots for the detection of silver ions. Nanoscale 5, 4691–4695 (2013). - PubMed
-
- Qin L., He X., Chen L. & Zhang Y. Turn-on fluorescent sensing of glutathione s-transferase at near-infrared region based on FRET between gold nanoclusters and gold nanorods. ACS Appl. Mater. Interfaces 7, 5965–5971 (2015). - PubMed
-
- Enkin N., Sharon E., Golub E. & Willner I. Ag nanocluster/DNA hybrids: functional modules for the detection of nitroaromatic and RDX explosives. Nano Lett. 14, 4918–4922 (2014). - PubMed
-
- Xie S., Tsunoyama H., Kurashige W., Negishi Y. & Tsukuda T. Enhancement in aerobic alcohol oxidation catalysis of Au25 clusters by single Pd atom doping. ACS Catal. 2, 1519–1523 (2012).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

